
PDF Publication Title:
Text from PDF Page: 008
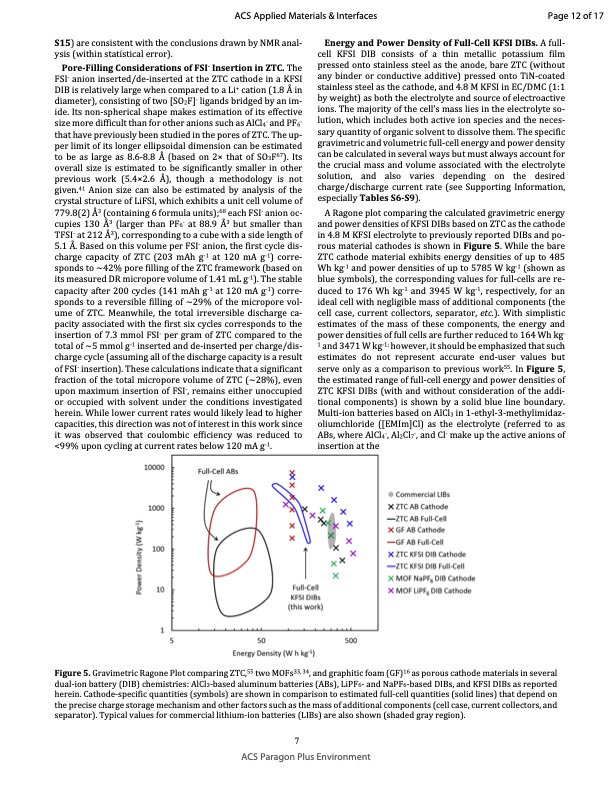
S15) are consistent with the conclusions drawn by NMR anal‐ ysis (within statistical error). Pore‐Filling Considerations of FSI‐ Insertion in ZTC. The FSI‐ anion inserted/de‐inserted at the ZTC cathode in a KFSI DIB is relatively large when compared to a Li+ cation (1.8 Å in diameter), consisting of two [SO2F]‐ ligands bridged by an im‐ ide. Its non‐spherical shape makes estimation of its effective size more difficult than for other anions such as AlCl4‐ and PF6‐ that have previously been studied in the pores of ZTC. The up‐ per limit of its longer ellipsoidal dimension can be estimated to be as large as 8.6‐8.8 Å (based on 2× that of SO3F67). Its overall size is estimated to be significantly smaller in other previous work (5.4×2.6 Å), though a methodology is not given.41 Anion size can also be estimated by analysis of the crystal structure of LiFSI, which exhibits a unit cell volume of 779.8(2) Å3 (containing 6 formula units);68 each FSI‐ anion oc‐ cupies 130 Å3 (larger than PF6‐ at 88.9 Å3 but smaller than TFSI‐ at 212 Å3), corresponding to a cube with a side length of 5.1 Å. Based on this volume per FSI‐ anion, the first cycle dis‐ charge capacity of ZTC (203 mAh g‐1 at 120 mA g‐1) corre‐ sponds to ~42% pore filling of the ZTC framework (based on its measured DR micropore volume of 1.41 mL g‐1). The stable capacity after 200 cycles (141 mAh g‐1 at 120 mA g‐1) corre‐ sponds to a reversible filling of ~29% of the micropore vol‐ ume of ZTC. Meanwhile, the total irreversible discharge ca‐ pacity associated with the first six cycles corresponds to the insertion of 7.3 mmol FSI‐ per gram of ZTC compared to the total of ~5 mmol g‐1 inserted and de‐inserted per charge/dis‐ charge cycle (assuming all of the discharge capacity is a result of FSI‐ insertion). These calculations indicate that a significant fraction of the total micropore volume of ZTC (~28%), even upon maximum insertion of FSI‐, remains either unoccupied or occupied with solvent under the conditions investigated herein. While lower current rates would likely lead to higher capacities, this direction was not of interest in this work since it was observed that coulombic efficiency was reduced to <99% upon cycling at current rates below 120 mA g‐1. Energy and Power Density of Full‐Cell KFSI DIBs. A full‐ cell KFSI DIB consists of a thin metallic potassium film pressed onto stainless steel as the anode, bare ZTC (without any binder or conductive additive) pressed onto TiN‐coated stainless steel as the cathode, and 4.8 M KFSI in EC/DMC (1:1 by weight) as both the electrolyte and source of electroactive ions. The majority of the cell’s mass lies in the electrolyte so‐ lution, which includes both active ion species and the neces‐ sary quantity of organic solvent to dissolve them. The specific gravimetric and volumetric full‐cell energy and power density can be calculated in several ways but must always account for the crucial mass and volume associated with the electrolyte solution, and also varies depending on the desired charge/discharge current rate (see Supporting Information, especially Tables S6‐S9). A Ragone plot comparing the calculated gravimetric energy and power densities of KFSI DIBs based on ZTC as the cathode in 4.8 M KFSI electrolyte to previously reported DIBs and po‐ rous material cathodes is shown in Figure 5. While the bare ZTC cathode material exhibits energy densities of up to 485 Wh kg‐1 and power densities of up to 5785 W kg‐1 (shown as blue symbols), the corresponding values for full‐cells are re‐ duced to 176 Wh kg‐1 and 3945 W kg‐1, respectively, for an ideal cell with negligible mass of additional components (the cell case, current collectors, separator, etc.). With simplistic estimates of the mass of these components, the energy and power densities of full cells are further reduced to 164 Wh kg‐ 1 and 3471 W kg‐1; however, it should be emphasized that such estimates do not represent accurate end‐user values but serve only as a comparison to previous work55. In Figure 5, the estimated range of full‐cell energy and power densities of ZTC KFSI DIBs (with and without consideration of the addi‐ tional components) is shown by a solid blue line boundary. Multi‐ion batteries based on AlCl3 in 1‐ethyl‐3‐methylimidaz‐ oliumchloride ([EMIm]Cl) as the electrolyte (referred to as ABs, where AlCl4‐, Al2Cl7‐, and Cl‐ make up the active anions of insertion at the ACS Applied Materials & Interfaces Page 12 of 17 Figure 5. Gravimetric Ragone Plot comparing ZTC,55 two MOFs33, 34, and graphitic foam (GF)16 as porous cathode materials in several dual‐ion battery (DIB) chemistries: AlCl3‐based aluminum batteries (ABs), LiPF6‐ and NaPF6‐based DIBs, and KFSI DIBs as reported herein. Cathode‐specific quantities (symbols) are shown in comparison to estimated full‐cell quantities (solid lines) that depend on the precise charge storage mechanism and other factors such as the mass of additional components (cell case, current collectors, and separator). Typical values for commercial lithium‐ion batteries (LIBs) are also shown (shaded gray region). 7 ACS Paragon Plus EnvironmentPDF Image | Zeolite-Templated Carbon as the Cathode
PDF Search Title:
Zeolite-Templated Carbon as the CathodeOriginal File Name Searched:
Stadie_ACSAMI_2019_FINAL.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |