
PDF Publication Title:
Text from PDF Page: 006
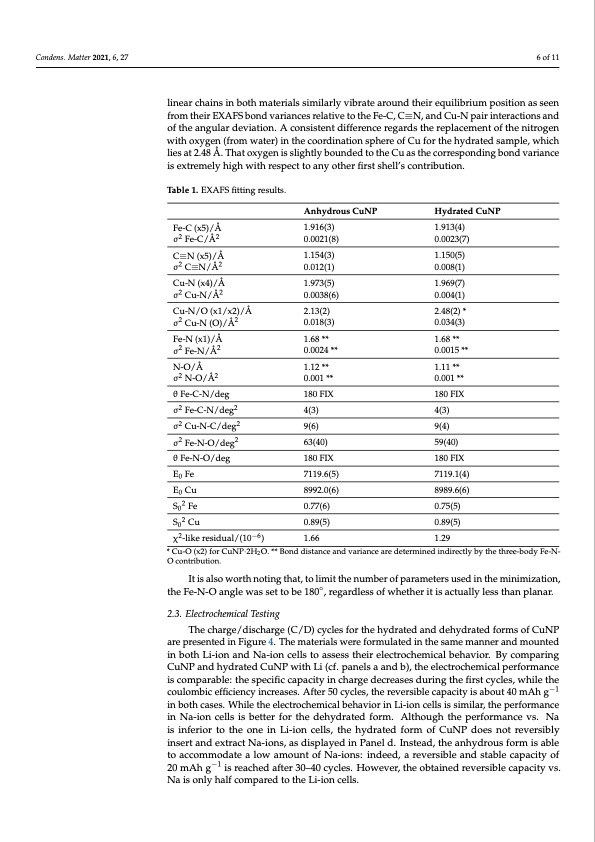
Condens. Matter 2021, 6, 27 6 of 11 linear chains in both materials similarly vibrate around their equilibrium position as seen from their EXAFS bond variances relative to the Fe-C, C≡N, and Cu-N pair interactions and of the angular deviation. A consistent difference regards the replacement of the nitrogen with oxygen (from water) in the coordination sphere of Cu for the hydrated sample, which lies at 2.48 Å. That oxygen is slightly bounded to the Cu as the corresponding bond variance is extremely high with respect to any other first shell’s contribution. Table 1. EXAFS fitting results. Fe-C (x5)/Å σ2 Fe-C/Å2 C≡N (x5)/Å σ2 C≡N/Å2 Cu-N (x4)/Å σ2 Cu-N/Å2 Cu-N/O (x1/x2)/Å σ2 Cu-N (O)/Å2 Fe-N (x1)/Å σ2 Fe-N/Å2 N-O/Å σ2 N-O/Å2 θ Fe-C-N/deg σ2 Fe-C-N/deg2 σ2 Cu-N-C/deg2 σ2 Fe-N-O/deg2 θ Fe-N-O/deg E0 Fe E0 Cu S02 Fe S02 Cu Anhydrous CuNP 1.916(3) 0.0021(8) 1.154(3) 0.012(1) 1.973(5) 0.0038(6) 2.13(2) 0.018(3) 1.68 ** 0.0024 ** 1.12 ** 0.001 ** 180 FIX 4(3) 9(6) 63(40) 180 FIX 7119.6(5) 8992.0(6) 0.77(6) 0.89(5) 1.66 Hydrated CuNP 1.913(4) 0.0023(7) 1.150(5) 0.008(1) 1.969(7) 0.004(1) 2.48(2) * 0.034(3) 1.68 ** 0.0015 ** 1.11 ** 0.001 ** 180 FIX 4(3) 9(4) 59(40) 180 FIX 7119.1(4) 8989.6(6) 0.75(5) 0.89(5) 1.29 χ2-like residual/(10−6) * Cu-O (x2) for CuNP·2H2O. ** Bond distance and variance are determined indirectly by the three-body Fe-N- O contribution. It is also worth noting that, to limit the number of parameters used in the minimization, the Fe-N-O angle was set to be 180◦, regardless of whether it is actually less than planar. 2.3. Electrochemical Testing The charge/discharge (C/D) cycles for the hydrated and dehydrated forms of CuNP are presented in Figure 4. The materials were formulated in the same manner and mounted in both Li-ion and Na-ion cells to assess their electrochemical behavior. By comparing CuNP and hydrated CuNP with Li (cf. panels a and b), the electrochemical performance is comparable: the specific capacity in charge decreases during the first cycles, while the coulombic efficiency increases. After 50 cycles, the reversible capacity is about 40 mAh g−1 in both cases. While the electrochemical behavior in Li-ion cells is similar, the performance in Na-ion cells is better for the dehydrated form. Although the performance vs. Na is inferior to the one in Li-ion cells, the hydrated form of CuNP does not reversibly insert and extract Na-ions, as displayed in Panel d. Instead, the anhydrous form is able to accommodate a low amount of Na-ions: indeed, a reversible and stable capacity of 20 mAh g−1 is reached after 30–40 cycles. However, the obtained reversible capacity vs. Na is only half compared to the Li-ion cells.PDF Image | Cross-Investigation on Copper Nitroprusside: Combining XRD and XAS
PDF Search Title:
Cross-Investigation on Copper Nitroprusside: Combining XRD and XASOriginal File Name Searched:
condensedmatter-06-00027-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |