
PDF Publication Title:
Text from PDF Page: 007
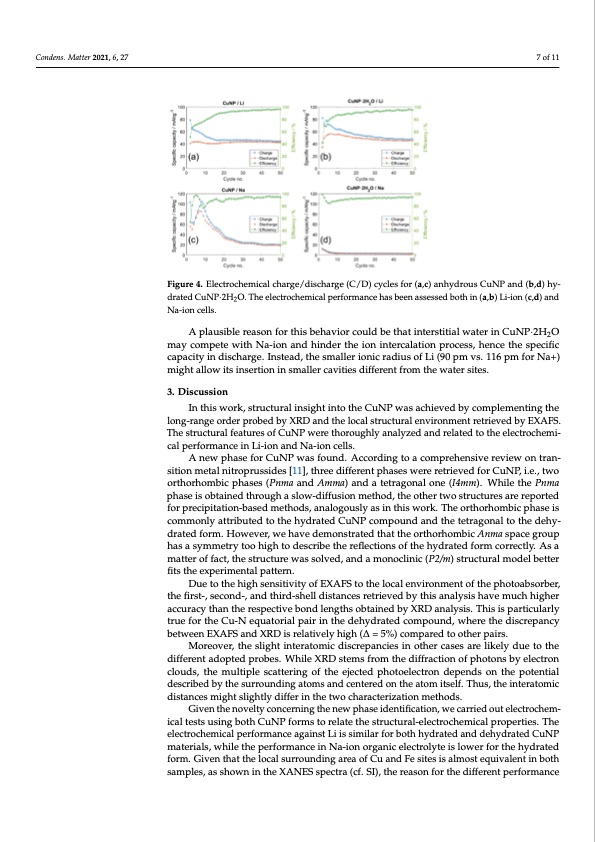
is able to accommodate a low amount of Na-ions: indeed, a reversible and stable capacity Condens. Matter 2021, 6, 27 of 20 mAh g−1 is reached after 30–40 cycles. However, the obtained reversible capacity vs. Na is only half compared to the Li-ion cells. A plausible reason for this behavior could be that interstitial water in CuNP∙2H2O may compete with Na-ion and hinder the ion intercalation process, hence the specific ca- 7 of 11 Figure 4. Electrochemical charge/discharge (C/D) cycles for (a,c) anhydrous CuNP and (b,d) hy- Figure 4. Electrochemical charge/discharge (C/D) cycles for (a,c) anhydrous CuNP and (b,d) hy- drated CuNP·2H O. The electrochemical performance has been assessed both in (a,b) Li-ion (c,d) and drated CuNP∙2H2O. T2he electrochemical performance has been assessed both in (a,b) Li-ion (c,d) pacity in discharge. Instead, the smaller ionic radius of Li (90 pm vs. 116 pm for Na+) might allow its insertion in smaller cavities different from the water sites. Na-ion cells. and Na-ion cells. A plausible reason for this behavior could be that interstitial water in CuNP·2H2O 3. Discussion may compete with Na-ion and hinder the ion intercalation process, hence the specific In this work, structural insight into the CuNP was achieved by complementing the capacity in discharge. Instead, the smaller ionic radius of Li (90 pm vs. 116 pm for Na+) long-range order probed by XRD and the local structural environment retrieved by EX- might allow its insertion in smaller cavities different from the water sites. AFS. The structural features of CuNP were thoroughly analyzed and related to the elec- trochemical performance in Li-ion and Na-ion cells. 3. Discussion A new phase for CuNP was found. According to a comprehensive review on transi- In this work, structural insight into the CuNP was achieved by complementing the tion metal nitroprussides [11], three different phases were retrieved for CuNP, i.e., two long-range order probed by XRD and the local structural environment retrieved by EXAFS. orthorhombic phases (Pnma and Amma) and a tetragonal one (I4mm). While the Pnma The structural features of CuNP were thoroughly analyzed and related to the electrochemi- phase is obtained through a slow-diffusion method, the other two structures are reported cal performance in Li-ion and Na-ion cells. for precipitation-based methods, analogously as in this work. The orthorhombic phase is A new phase for CuNP was found. According to a comprehensive review on tran- commonly attributed to the hydrated CuNP compound and the tetragonal to the dehy- sition metal nitroprussides [11], three different phases were retrieved for CuNP, i.e., two drated form. However, we have demonstrated that the orthorhombic Anma space group orthorhombic phases (Pnma and Amma) and a tetragonal one (I4mm). While the Pnma has a symmetry too high to describe the reflections of the hydrated form correctly. As a phase is obtained through a slow-diffusion method, the other two structures are reported matter of fact, the structure was solved, and a monoclinic (P2/m) structural model better for precipitation-based methods, analogously as in this work. The orthorhombic phase is fits the experimental pattern. commonly attributed to the hydrated CuNP compound and the tetragonal to the dehy- Due to the high sensitivity of EXAFS to the local environment of the photoabsorber, drated form. However, we have demonstrated that the orthorhombic Anma space group the first-, second-, and third-shell distances retrieved by this analysis have much higher has a symmetry too high to describe the reflections of the hydrated form correctly. As a accuracy than the respective bond lengths obtained by XRD analysis. This is particularly matter of fact, the structure was solved, and a monoclinic (P2/m) structural model better true for the Cu-N equatorial pair in the dehydrated compound, where the discrepancy fits the experimental pattern. between EDXuAeFtSo athnedhXigRhDsiesnrseiltaivtivtyeloyfhEigXhA(FΔS=to5%th)eclomcaplaernevdirtonomtheenrtpoafitrhs.e photoabsorber, the first-, second-, and third-shell distances retrieved by this analysis have much higher accuracy than the respective bond lengths obtained by XRD analysis. This is particularly true for the Cu-N equatorial pair in the dehydrated compound, where the discrepancy between EXAFS and XRD is relatively high (∆ = 5%) compared to other pairs. Moreover, the slight interatomic discrepancies in other cases are likely due to the different adopted probes. While XRD stems from the diffraction of photons by electron clouds, the multiple scattering of the ejected photoelectron depends on the potential described by the surrounding atoms and centered on the atom itself. Thus, the interatomic distances might slightly differ in the two characterization methods. Given the novelty concerning the new phase identification, we carried out electrochem- ical tests using both CuNP forms to relate the structural-electrochemical properties. The electrochemical performance against Li is similar for both hydrated and dehydrated CuNP materials, while the performance in Na-ion organic electrolyte is lower for the hydrated form. Given that the local surrounding area of Cu and Fe sites is almost equivalent in both samples, as shown in the XANES spectra (cf. SI), the reason for the different performancePDF Image | Cross-Investigation on Copper Nitroprusside: Combining XRD and XAS
PDF Search Title:
Cross-Investigation on Copper Nitroprusside: Combining XRD and XASOriginal File Name Searched:
condensedmatter-06-00027-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |