
PDF Publication Title:
Text from PDF Page: 007
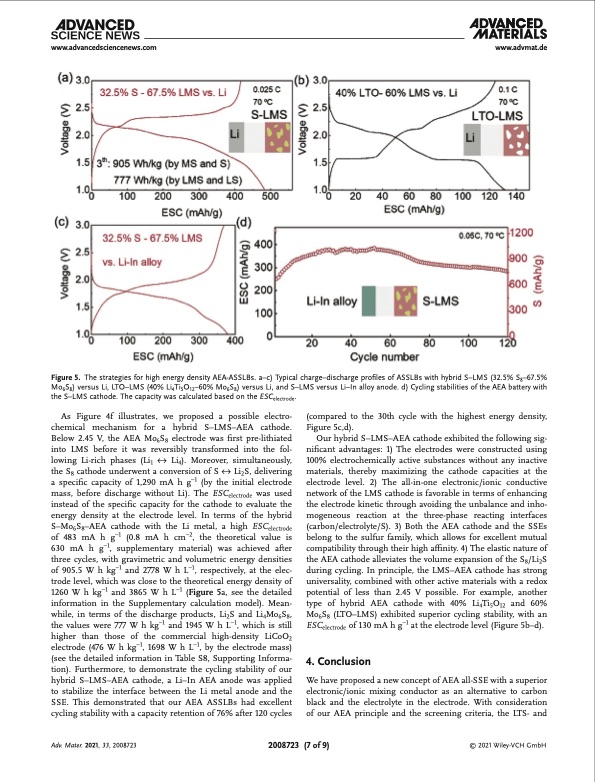
www.advancedsciencenews.com www.advmat.de Figure 5. The strategies for high energy density AEA-ASSLBs. a–c) Typical charge–discharge profiles of ASSLBs with hybrid S–LMS (32.5% S8–67.5% Mo6S8) versus Li, LTO–LMS (40% Li4Ti5O12–60% Mo6S8) versus Li, and S–LMS versus Li–In alloy anode. d) Cycling stabilities of the AEA battery with the S–LMS cathode. The capacity was calculated based on the ESCelectrode. As Figure 4f illustrates, we proposed a possible electro- chemical mechanism for a hybrid S–LMS–AEA cathode. Below 2.45 V, the AEA Mo6S8 electrode was first pre-lithiated into LMS before it was reversibly transformed into the fol- lowing Li-rich phases (Li1 ↔ Li4). Moreover, simultaneously, the S8 cathode underwent a conversion of S ↔ Li2S, delivering a specific capacity of 1,290 mA h g−1 (by the initial electrode mass, before discharge without Li). The ESCelectrode was used instead of the specific capacity for the cathode to evaluate the energy density at the electrode level. In terms of the hybrid S–Mo6S8–AEA cathode with the Li metal, a high ESCelectrode of 483 mA h g−1 (0.8 mA h cm−2, the theoretical value is 630 mA h g−1, supplementary material) was achieved after three cycles, with gravimetric and volumetric energy densities of 905.5 W h kg−1 and 2778 W h L−1, respectively, at the elec- trode level, which was close to the theoretical energy density of 1260 W h kg−1 and 3865 W h L−1 (Figure 5a, see the detailed information in the Supplementary calculation model). Mean- while, in terms of the discharge products, Li2S and Li4Mo6S8, the values were 777 W h kg−1 and 1945 W h L−1, which is still higher than those of the commercial high-density LiCoO2 electrode (476 W h kg−1, 1698 W h L−1, by the electrode mass) (see the detailed information in Table S8, Supporting Informa- tion). Furthermore, to demonstrate the cycling stability of our hybrid S–LMS–AEA cathode, a Li–In AEA anode was applied to stabilize the interface between the Li metal anode and the SSE. This demonstrated that our AEA ASSLBs had excellent cycling stability with a capacity retention of 76% after 120 cycles (compared to the 30th cycle with the highest energy density, Figure5c,d). Our hybrid S–LMS–AEA cathode exhibited the following sig- nificant advantages: 1) The electrodes were constructed using 100% electrochemically active substances without any inactive materials, thereby maximizing the cathode capacities at the electrode level. 2) The all-in-one electronic/ionic conductive network of the LMS cathode is favorable in terms of enhancing the electrode kinetic through avoiding the unbalance and inho- mogeneous reaction at the three-phase reacting interfaces (carbon/electrolyte/S). 3) Both the AEA cathode and the SSEs belong to the sulfur family, which allows for excellent mutual compatibility through their high affinity. 4) The elastic nature of the AEA cathode alleviates the volume expansion of the S8/Li2S during cycling. In principle, the LMS–AEA cathode has strong universality, combined with other active materials with a redox potential of less than 2.45 V possible. For example, another type of hybrid AEA cathode with 40% Li4Ti5O12 and 60% Mo6S8 (LTO–LMS) exhibited superior cycling stability, with an ESCelectrode of 130 mA h g−1 at the electrode level (Figure 5b–d). 4. Conclusion We have proposed a new concept of AEA all-SSE with a superior electronic/ionic mixing conductor as an alternative to carbon black and the electrolyte in the electrode. With consideration of our AEA principle and the screening criteria, the LTS- and Adv. Mater. 2021, 33, 2008723 2008723 (7 of 9) © 2021 Wiley-VCH GmbHPDF Image | Dense All-Electrochem-Active Electrodes for All-Solid-State Lithium Batteries
PDF Search Title:
Dense All-Electrochem-Active Electrodes for All-Solid-State Lithium BatteriesOriginal File Name Searched:
Li21LiuAM.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |