
PDF Publication Title:
Text from PDF Page: 004
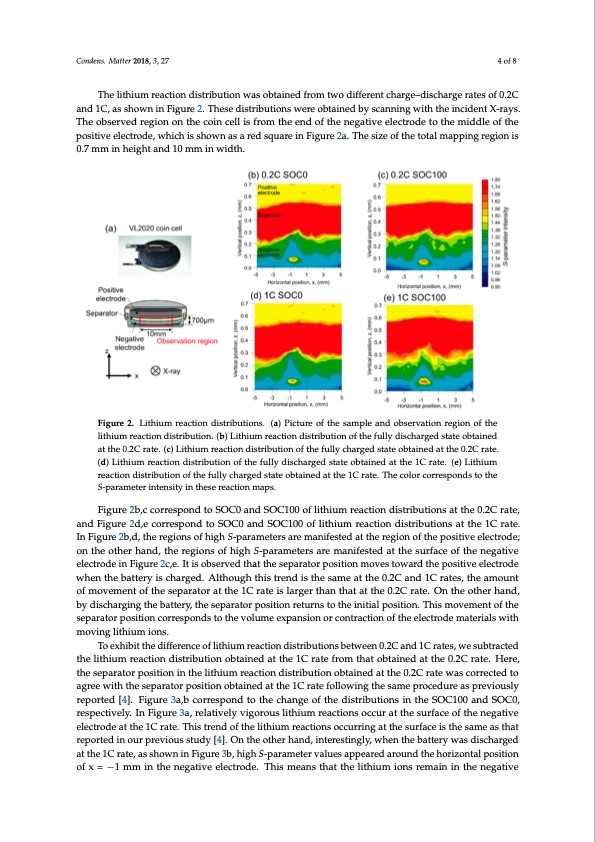
Condens. Matter 2018, 3, x FOR PEER REVIEW 4 of 8 Figure 1. Internal structure of the VL2020 coin cell observed by the S-parameters. (a) Components of the coin cell are distinguished by the value of S-parameters. (b) Change of S-parameters at positive and negative electrodes with the charge–discharge cycle displayed. The fully charged state (state of charge (SOC) 100) and fully discharged state (SOC0) are shown in red and blue circles, respectively. Condens. Matter 2018, 3, 27 The background color shows the regions of the corresponding components of the coin cell. 4 of 8 TThheelitlhitihuimumreraecaticotinondisdtirsitbruibtuiotniownawsaosbtoabintaeidnefdrofmrotmwotwdoiffderieffnetrecnhtarcghea–rdgies–cdhiasrcgheargateesraotfes0.2oCf an0d.2C1Ca,nads1shCo,wasnsihnoFwignuirneF2i.gTuhres2e. TdhisetrsiebduitsiotrnibsuwtieornesowbtearieneodbtbayinsecdanbnyinscgawnnitihngthweiitnhctihdenintcXid-reanyts. TXhe-raoybs.eTrvhedobresegrivoendornegthioenconinthcelcloisnfcreolmlisthfreomentdheofenthdeonfethgeatnivegeaetliveceteroledcetrotodethteomthiedmdlieddolfetohfe pothseitipvoeseitlievcetroeldeec,trwohdiec,hwishischhowisnshaoswanreadssaquraerdesiqnuFairgeuirne 2Faig. uTrhee2saiz.eTohfethsiezetootafltmheaptoptianlgmraegpipoingis 0.r7egmiomn ins 0h.e7igmhmt aindh1e0igmhtmanind w10idmthm. in width. FiFgiugurere2.2.LLitihthiuiumrreeaaccttiioondiissttrriibuttiions. (a) Picture of the samplle and obserrvattiioonnrreegioionnoofftthee litlihthiuiummreraecatcitoionnddisitsrtirbibuutitoionn. .((b))Liitthiium reactiion distribution of the fully discharged statte obttaiineed ataththee0.02.C2Craratete..(c(c))LLitihthiuiumrreeaaccttiiondiistributionofthefullychargedstatteobttaaiineeddaatttthhee00.2.2CCraratete.. (d()d)LiLtihtihuiummreraecatcitoionnddisitsrtirbibuutitoionnoofftthheeffuulllydiissccharged sttate obtained at the 1C rate. (e)) Liitthiium reraecatcitoionnddisitsrtirbibuutitoionnooffththeefufullylycchhaarrgeedssttatteobttained at the 1C rate. The color correspondssttotthee S-Sp-apraarmameteetrerinintetnensistiytyininththeeseserereaacctitoionnmaappss. . Figure 2b,c correspond to SOC0 and SOC100 of lithium reaction distributions at the 0.2C rate, Figure 2b,c correspond to SOC0 and SOC100 of lithium reaction distributions at the 0.2C rate, and Figure 2d,e correspond to SOC0 and SOC100 of lithium reaction distributions at the 1C rate. and Figure 2d,e correspond to SOC0 and SOC100 of lithium reaction distributions at the 1C rate. In In Figure 2b,d, the regions of high S-parameters are manifested at the region of the positive electrode; Figure 2b,d, the regions of high S-parameters are manifested at the region of the positive electrode; ononththeeooththererhhaanndd, ,ththeerereggioionnssooffhhigighS--parrameters are manifested at the surface off the negattiivee elelcetcrtordodeeiniFnigFuigreur2ec,e2.cI,et.isItobiserovbesdertvheadt ttheastetphaerasteopraproastoitriopnomsiotivoens tmowovaersd thoewparodsittihve epleocstirtoivdee wehlecntrtohdeebwathternythisecbhaatrtegreydi.sAchltahroguegdh. Athltihsoturegnhdthisisthtrensadmisetahtetshaem0e.2aCt tahned01.2Cranteds1, Cthreaatems,otuhnet ofamouvnetmoefnmtovfetmhensetpoafrtahteosreaptatrhaeto1rCatrtahte1isClarragteristhlarngetrhathtantthaet0a.2tCthera0t.e2.COrnatteh.eOontthheerohtahnedr, byhadnidsc,hbarygidnigscthaerbgainttgeryth,tehebsaettpearrya,tothreposeiptiaornatroerturpnosittoiotnherientiutiranlspotosittihone.iTnhitiisamlopvoesimtieont.oTfhthise sempoarvaetmorenptosoitfiothnecosrerpeasrpaotnordsptositthioenvoclourmreespeoxnpdansstionthoer cvoonlutrmacetioenxpoafntshieoneleocrtrcoodnetrmacattieorniaolsfwthiteh meolevcitnrgodliethmiuamteriioanls.with moving lithium ions. ToToexehxibhitbtihtethdeiffderieffnecrenocfelitohfiulmithrieuamctiorenadctiisotrnibduitsiotrnibsubteiotwnseebne0t.w2Ceeannd0.12Cranteds,1wCesruabtetsr,acwteed subtracted the lithium reaction distribution obtained at the 1C rate from that obtained at the 0.2C the lithium reaction distribution obtained at the 1C rate from that obtained at the 0.2C rate. Here, rate. Here, the separator position in the lithium reaction distribution obtained at the 0.2C rate was the separator position in the lithium reaction distribution obtained at the 0.2C rate was corrected to corrected to agree with the separator position obtained at the 1C rate following the same procedure agree with the separator position obtained at the 1C rate following the same procedure as previously as previously reported [4]. Figure 3a,b correspond to the change of the distributions in the SOC100 reported [4]. Figure 3a,b correspond to the change of the distributions in the SOC100 and SOC0, respectively. In Figure 3a, relatively vigorous lithium reactions occur at the surface of the negative electrode at the 1C rate. This trend of the lithium reactions occurring at the surface is the same as that reported in our previous study [4]. On the other hand, interestingly, when the battery was discharged at the 1C rate, as shown in Figure 3b, high S-parameter values appeared around the horizontal position of x = −1 mm in the negative electrode. This means that the lithium ions remain in the negativePDF Image | Dependency of the Charge–Discharge Rate on Lithium Reaction Distributions
PDF Search Title:
Dependency of the Charge–Discharge Rate on Lithium Reaction DistributionsOriginal File Name Searched:
condensedmatter-03-00027.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |