
PDF Publication Title:
Text from PDF Page: 003
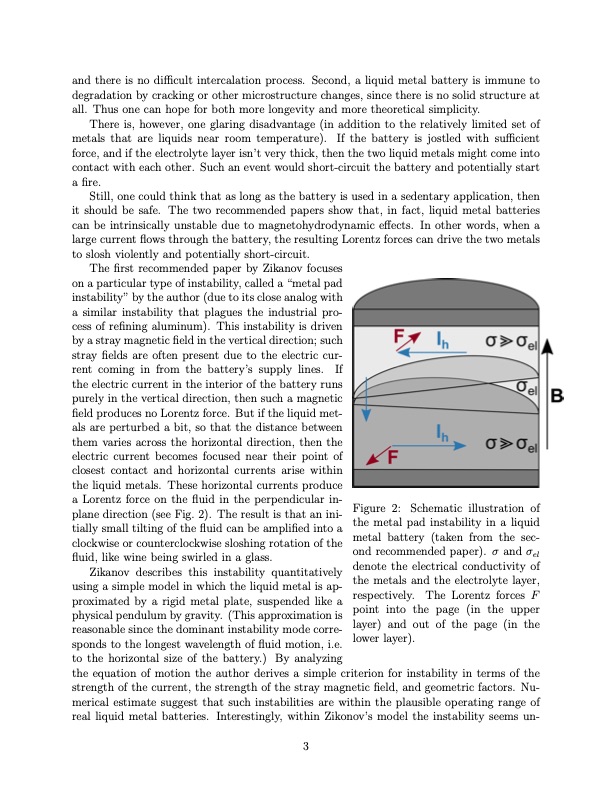
and there is no difficult intercalation process. Second, a liquid metal battery is immune to degradation by cracking or other microstructure changes, since there is no solid structure at all. Thus one can hope for both more longevity and more theoretical simplicity. There is, however, one glaring disadvantage (in addition to the relatively limited set of metals that are liquids near room temperature). If the battery is jostled with sufficient force, and if the electrolyte layer isn’t very thick, then the two liquid metals might come into contact with each other. Such an event would short-circuit the battery and potentially start a fire. Still, one could think that as long as the battery is used in a sedentary application, then it should be safe. The two recommended papers show that, in fact, liquid metal batteries can be intrinsically unstable due to magnetohydrodynamic effects. In other words, when a large current flows through the battery, the resulting Lorentz forces can drive the two metals to slosh violently and potentially short-circuit. The first recommended paper by Zikanov focuses on a particular type of instability, called a “metal pad instability” by the author (due to its close analog with a similar instability that plagues the industrial pro- cess of refining aluminum). This instability is driven by a stray magnetic field in the vertical direction; such stray fields are often present due to the electric cur- rent coming in from the battery’s supply lines. If the electric current in the interior of the battery runs purely in the vertical direction, then such a magnetic field produces no Lorentz force. But if the liquid met- als are perturbed a bit, so that the distance between them varies across the horizontal direction, then the electric current becomes focused near their point of closest contact and horizontal currents arise within the liquid metals. These horizontal currents produce a Lorentz force on the fluid in the perpendicular in- plane direction (see Fig. 2). The result is that an ini- tially small tilting of the fluid can be amplified into a clockwise or counterclockwise sloshing rotation of the fluid, like wine being swirled in a glass. Figure 2: Schematic illustration of the metal pad instability in a liquid metal battery (taken from the sec- ond recommended paper). σ and σel denote the electrical conductivity of the metals and the electrolyte layer, respectively. The Lorentz forces F point into the page (in the upper layer) and out of the page (in the lower layer). Zikanov describes this instability quantitatively using a simple model in which the liquid metal is ap- proximated by a rigid metal plate, suspended like a physical pendulum by gravity. (This approximation is reasonable since the dominant instability mode corre- sponds to the longest wavelength of fluid motion, i.e. to the horizontal size of the battery.) By analyzing the equation of motion the author derives a simple criterion for instability in terms of the strength of the current, the strength of the stray magnetic field, and geometric factors. Nu- merical estimate suggest that such instabilities are within the plausible operating range of real liquid metal batteries. Interestingly, within Zikonov’s model the instability seems un- 3PDF Image | Liquid metal batteries and their magnetohydrodynamic instabilities
PDF Search Title:
Liquid metal batteries and their magnetohydrodynamic instabilitiesOriginal File Name Searched:
JCCM_January_2022_02.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |