
PDF Publication Title:
Text from PDF Page: 006
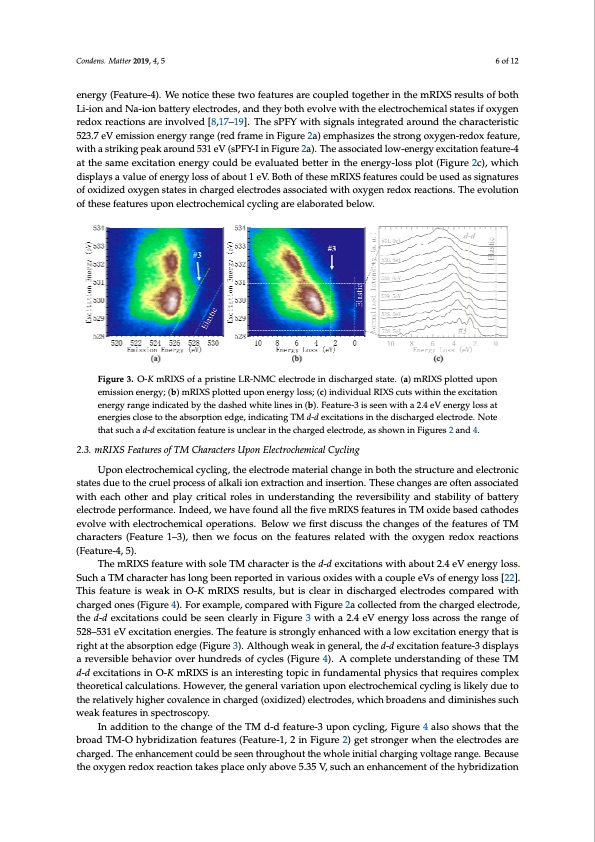
Condens. Matter 2019, 4, x FOR PEER REVIEW 6 of 12 Fourth, two associated mRIXS features emerged in the charged electrodes if oxygen redox Condens. Matter 2019, 4, 5 6 of 12 reactions were involved [8,17–19]. One is a sharp feature at a 523.7 eV emission and 531 eV excitation energy (Feature-5), while the other is a weak feature close to the elastic line at the same excitation energy (Feature-4). We notice these two features are coupled together in the mRIXS results of both energy (Feature-4). We notice these two features are coupled together in the mRIXS results of both Li-ion and Na-ion battery electrodes, and they both evolve with the electrochemical states if oxygen Li-ion and Na-ion battery electrodes, and they both evolve with the electrochemical states if oxygen redox reactions are involved [8,17–19]. The sPFY with signals integrated around the characteristic redox reactions are involved [8,17–19]. The sPFY with signals integrated around the characteristic 523.7 eV emission energy range (red frame in Figure 2a) emphasizes the strong oxygen-redox feature, 523.7 eV emission energy range (red frame in Figure 2a) emphasizes the strong oxygen-redox with a striking peak around 531 eV (sPFY-I in Figure 2a). The associated low-energy excitation feature-4 feature, with a striking peak around 531 eV (sPFY-I in Figure 2a). The associated low-energy at the same excitation energy could be evaluated better in the energy-loss plot (Figure 2c), which excitation feature-4 at the same excitation energy could be evaluated better in the energy-loss plot displays a value of energy loss of about 1 eV. Both of these mRIXS features could be used as signatures (Figure 2c), which displays a value of energy loss of about 1 eV. Both of these mRIXS features could of oxidized oxygen states in charged electrodes associated with oxygen redox reactions. The evolution be used as signatures of oxidized oxygen states in charged electrodes associated with oxygen redox of these features upon electrochemical cycling are elaborated below. reactions. The evolution of these features upon electrochemical cycling are elaborated below. Figure 3. O-K mRIXS of a pristine LR-NMC electrode in discharged state. (a) mRIXS plotted upon Figure 3. O-K mRIXS of a pristine LR-NMC electrode in discharged state. (a) mRIXS plotted upon emission energy; (b) mRIXS plotted upon energy loss; (c) individual RIXS cuts within the excitation emission energy; (b) mRIXS plotted upon energy loss; (c) individual RIXS cuts within the excitation energy range indicated by the dashed white lines in (b). Feature-3 is seen with a 2.4 eV energy loss at energy range indicated by the dashed white lines in (b). Feature-3 is seen with a 2.4 eV energy loss at energies close to the absorption edge, indicating TM d-d excitations in the discharged electrode. Note energies close to the absorption edge, indicating TM d-d excitations in the discharged electrode. Note that such a d-d excitation feature is unclear in the charged electrode, as shown in Figures 2 and 4. that such a d-d excitation feature is unclear in the charged electrode, as shown in Figures 2 and 4. 2.3. mRIXS Features of TM Characters Upon Electrochemical Cycling 2.3. mRIXS Features of TM Characters Upon Electrochemical Cycling Upon electrochemical cycling, the electrode material change in both the structure and electronic states due to the cruel process of alkali ion extraction and insertion. These changes are often associated Upon electrochemical cycling, the electrode material change in both the structure and electronic with each other and play critical roles in understanding the reversibility and stability of battery states due to the cruel process of alkali ion extraction and insertion. These changes are often electrode performance. Indeed, we have found all the five mRIXS features in TM oxide based cathodes associated with each other and play critical roles in understanding the reversibility and stability of evolve with electrochemical operations. Below we first discuss the changes of the features of TM battery electrode performance. Indeed, we have found all the five mRIXS features in TM oxide based characters (Feature 1–3), then we focus on the features related with the oxygen redox reactions cathodes evolve with electrochemical operations. Below we first discuss the changes of the features (Feature-4, 5). of TM characters (Feature 1–3), then we focus on the features related with the oxygen redox reactions The mRIXS feature with sole TM character is the d-d excitations with about 2.4 eV energy loss. (Feature-4, 5). Such a TM character has long been reported in various oxides with a couple eVs of energy loss [22]. The mRIXS feature with sole TM character is the d-d excitations with about 2.4 eV energy loss. This feature is weak in O-K mRIXS results, but is clear in discharged electrodes compared with Such a TM character has long been reported in various oxides with a couple eVs of energy loss [22]. charged ones (Figure 4). For example, compared with Figure 2a collected from the charged electrode, This feature is weak in O-K mRIXS results, but is clear in discharged electrodes compared with the d-d excitations could be seen clearly in Figure 3 with a 2.4 eV energy loss across the range of charged ones (Figure 4). For example, compared with Figure 2d collected from the charged 528–531 eV excitation energies. The feature is strongly enhanced with a low excitation energy that is electrode, the d-d excitations could be seen clearly in Figure 3 with a 2.4 eV energy loss across the right at the absorption edge (Figure 3). Although weak in general, the d-d excitation feature-3 displays range of 528–531 eV excitation energies. The feature is strongly enhanced with a low excitation a reversible behavior over hundreds of cycles (Figure 4). A complete understanding of these TM energy that is right at the absorption edge (Figure 3). Although weak in general, the d-d excitation d-d excitations in O-K mRIXS is an interesting topic in fundamental physics that requires complex feature-3 displays a reversible behavior over hundreds of cycles (Figure 4). A complete theoretical calculations. However, the general variation upon electrochemical cycling is likely due to understanding of these TM d-d excitations in O-K mRIXS is an interesting topic in fundamental the relatively higher covalence in charged (oxidized) electrodes, which broadens and diminishes such physics that requires complex theoretical calculations. However, the general variation upon weak features in spectroscopy. electrochemical cycling is likely due to the relatively higher covalence in charged (oxidized) In addition to the change of the TM d-d feature-3 upon cycling, Figure 4 also shows that the electrodes, which broadens and diminishes such weak features in spectroscopy. broad TM-O hybridization features (Feature-1, 2 in Figure 2) get stronger when the electrodes are In addition to the change of the TM d-d feature-3 upon cycling, Figure 4 also shows that the charged. The enhancement could be seen throughout the whole initial charging voltage range. Because broad TM-O hybridization features (Feature-1, 2 in Figure 2) get stronger when the electrodes are the oxygen redox reaction takes place only above 5.35 V, such an enhancement of the hybridizationPDF Image | Oxygen Redox Reactions in Batteries Resonant Inelastic X-ray Scattering
PDF Search Title:
Oxygen Redox Reactions in Batteries Resonant Inelastic X-ray ScatteringOriginal File Name Searched:
condensedmatter-04-00005.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |