
PDF Publication Title:
Text from PDF Page: 007
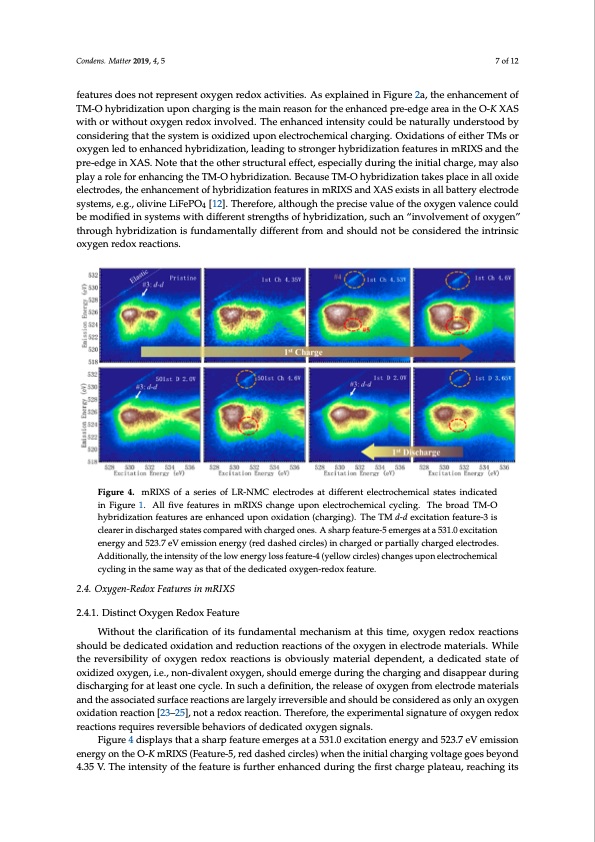
Condens. Matter 2019, 4, x FOR PEER REVIEW 7 of 12 Condens. Matter 2019, 4, 5 7 of 12 charged. The enhancement could be seen throughout the whole initial charging voltage range. Because the oxygen redox reaction takes place only above 5.35 V, such an enhancement of the features does not represent oxygen redox activities. As explained in Figure 2a, the enhancement of hybridization features does not represent oxygen redox activities. As explained in Figure 2a, the TM-O hybridization upon charging is the main reason for the enhanced pre-edge area in the O-K XAS enhancement of TM-O hybridization upon charging is the main reason for the enhanced pre-edge with or without oxygen redox involved. The enhanced intensity could be naturally understood by area in the O-K XAS with or without oxygen redox involved. The enhanced intensity could be considering that the system is oxidized upon electrochemical charging. Oxidations of either TMs or naturally understood by considering that the system is oxidized upon electrochemical charging. oxygen led to enhanced hybridization, leading to stronger hybridization features in mRIXS and the Oxidations of either TMs or oxygen led to enhanced hybridization, leading to stronger hybridization pre-edge in XAS. Note that the other structural effect, especially during the initial charge, may also features in mRIXS and the pre-edge in XAS. Note that the other structural effect, especially during play a role for enhancing the TM-O hybridization. Because TM-O hybridization takes place in all oxide the initial charge, may also play a role for enhancing the TM-O hybridization. Because TM-O electrodes, the enhancement of hybridization features in mRIXS and XAS exists in all battery electrode hybridization takes place in all oxide electrodes, the enhancement of hybridization features in systems, e.g., olivine LiFePO4 [12]. Therefore, although the precise value of the oxygen valence could mRIXS and XAS exists in all battery electrode systems, e.g., olivine LiFePO4 [12]. Therefore, although be modified in systems with different strengths of hybridization, such an “involvement of oxygen” the precise value of the oxygen valence could be modified in systems with different strengths of through hybridization is fundamentally different from and should not be considered the intrinsic hybridization, such an “involvement of oxygen” through hybridization is fundamentally different oxygen redox reactions. from and should not be considered the intrinsic oxygen redox reactions. Figure 4. mRIXS of a series of LR-NMC electrodes at different electrochemical states indicated Figure 4. mRIXS of a series of LR-NMC electrodes at different electrochemical states indicated in in Figure 1. All five features in mRIXS change upon electrochemical cycling. The broad TM-O Figure 1. All five features in mRIXS change upon electrochemical cycling. The broad TM-O hybridization features are enhanced upon oxidation (charging). The TM d-d excitation feature-3 is hybridization features are enhanced upon oxidation (charging). The TM d-d excitation feature-3 is clearer in discharged states compared with charged ones. A sharp feature-5 emerges at a 531.0 excitation clearer in discharged states compared with charged ones. A sharp feature-5 emerges at a 531.0 energy and 523.7 eV emission energy (red dashed circles) in charged or partially charged electrodes. excitation energy and 523.7 eV emission energy (red dashed circles) in charged or partially charged Additionally, the intensity of the low energy loss feature-4 (yellow circles) changes upon electrochemical electrodes. Additionally, the intensity of the low energy loss feature-4 (yellow circles) changes upon cycling in the same way as that of the dedicated oxygen-redox feature. electrochemical cycling in the same way as that of the dedicated oxygen-redox feature. 2.4. Oxygen-Redox Features in mRIXS 2.4. Oxygen-Redox Features in mRIXS 2.4.1. Distinct Oxygen Redox Feature 2.4.1. Distinct Oxygen Redox Feature Without the clarification of its fundamental mechanism at this time, oxygen redox reactions shoulWd ibtheoduetdtihcaetecdlaroixficdaatitoionnoafnidtsrefudnudctaimonenretalctmioencshoanf itshme oaxtytgheisn tiinmeel,ecotxroydgenmraetdeorixalrse.aWctihoinles tsheoureldvebresibdielditiycaotfedoxoyxgidenatiroendoaxndreraecdtiuocntisoins roebavctiiounsslyofmthateeroixayl gdeenpiendeleenct,roaddeemdiactaetreidals.taWtehiolef othxeidrizeevderosxibyiglietny, oi.fe.o, nxyogne-dnivraeldeonxt orxeyacgteinon, shiosuoldbveimouersglye dmuartienrgiatlhedecpheanrgdienngt,anadddeidsiacpaptedarsdtautreinogf doixsicdhiazregdinogxfyogreant,lei.aes.,t onnoen-cdyicvlea.leIntsuocxhygaedne,fisnhiotiuolnd, tehmeerergleasdeuorfinogxytghencfhroamrgienlegctarnodedmisatperpieaalsr adnudritnhgeadsisochciartgedinsgufrofarcaetrleeaacsttioonnsearceyclalerg.eInlysiurrcehvearsdiebflienaitniodns,htohueldrebleacsoenosifdoexreydgeansofrnolymaneleocxtyrgoedne omxaidteartiaolnsraenadctitohne[a2s3s–o2c5i]a,tnedotsaurefadcoexreactions. Tahrerleafrogrel,ythierrexvpeersrimblenatnaldsisghnoautuldreboefcooxnysgiedneredoaxs roenalcytioans roexqyugierens roexviderastibolne brehaactviioonrs[o2f3d–2e5d]i,cantoedt oaxyregdeonxsigrenacltsi.on. Therefore, the experimental signaFtiugruereof4odxiyspgelanyrsetdhoaxtaresahcatriopnfsearetuqrueireemserregvesrsaitbale5b3e1h.0aevxiocritsaotifodneedniecragteydaonxdy5g2e3n.7siegVnaelms.ission energFyigounrtehe4Od-KispmlaRyIsXSth(Faetaatusreh-a5r,predfedatausrhedemcirecrlgees)swahtean t5h3e1i.0niteixalcicthaatirogningenveorlgtaygeangdoes52b3e.y7oneVd 4e.m35isVsi.oTnhenienrtgeynsointythoef tOh-eKfematRuIrXeSis(Ffeuartuhreer-5e,nrheadndceadshdeudrcinirgcltehse) wfirhsetnchthaergieniptilaltecahuar,grienagchvionlgtaigtsePDF Image | Oxygen Redox Reactions in Batteries Resonant Inelastic X-ray Scattering
PDF Search Title:
Oxygen Redox Reactions in Batteries Resonant Inelastic X-ray ScatteringOriginal File Name Searched:
condensedmatter-04-00005.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |