
PDF Publication Title:
Text from PDF Page: 006
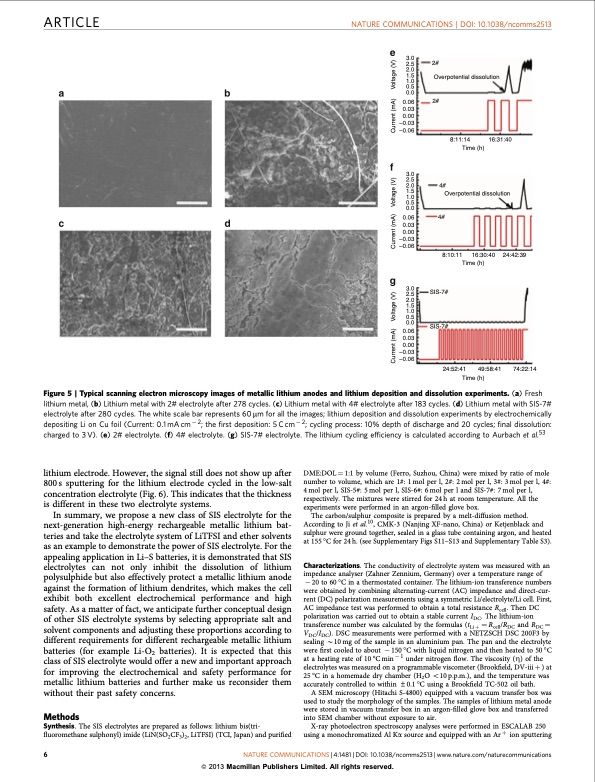
ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms2513 3.0 2.5 2.0 1.5 1.0 0.5 0.0 0.06 0.03 0.00 –0.03 –0.06 3.0 2.5 2.0 1.5 1.0 0.5 0.0 0.06 0.03 0.00 –0.03 –0.06 3.0 2.5 2.0 1.5 1.0 0.5 0.0 0.03 0.00 –0.03 –0.06 2# Overpotential dissolution 2# 4# 8:10:11 16:30:40 Time (h) SIS-7# 8:11:14 Time (h) 16:31:40 Figure 5 | Typical scanning electron microscopy images of metallic lithium anodes and lithium deposition and dissolution experiments. (a) Fresh lithium metal, (b) Lithium metal with 2# electrolyte after 278 cycles. (c) Lithium metal with 4# electrolyte after 183 cycles. (d) Lithium metal with SIS-7# electrolyte after 280 cycles. The white scale bar represents 60 mm for all the images; lithium deposition and dissolution experiments by electrochemically depositing Li on Cu foil (Current: 0.1 mA cm 2; the first deposition: 5 C cm 2; cycling process: 10% depth of discharge and 20 cycles; final dissolution: charged to 3 V). (e) 2# electrolyte. (f) 4# electrolyte. (g) SIS-7# electrolyte. The lithium cycling efficiency is calculated according to Aurbach et al.53 lithium electrode. However, the signal still does not show up after 800s sputtering for the lithium electrode cycled in the low-salt concentration electrolyte (Fig. 6). This indicates that the thickness is different in these two electrolyte systems. In summary, we propose a new class of SIS electrolyte for the next-generation high-energy rechargeable metallic lithium bat- teries and take the electrolyte system of LiTFSI and ether solvents as an example to demonstrate the power of SIS electrolyte. For the appealing application in Li–S batteries, it is demonstrated that SIS electrolytes can not only inhibit the dissolution of lithium polysulphide but also effectively protect a metallic lithium anode against the formation of lithium dendrites, which makes the cell exhibit both excellent electrochemical performance and high safety. As a matter of fact, we anticipate further conceptual design of other SIS electrolyte systems by selecting appropriate salt and solvent components and adjusting these proportions according to different requirements for different rechargeable metallic lithium batteries (for example Li-O2 batteries). It is expected that this class of SIS electrolyte would offer a new and important approach for improving the electrochemical and safety performance for metallic lithium batteries and further make us reconsider them without their past safety concerns. Methods Synthesis. The SIS electrolytes are prepared as follows: lithium bis(tri- fluoromethane sulphonyl) imide (LiN(SO2CF3)2, LiTFSI) (TCI, Japan) and purified DME:DOL 1⁄4 1:1 by volume (Ferro, Suzhou, China) were mixed by ratio of mole number to volume, which are 1#: 1 mol per l, 2#: 2 mol per l, 3#: 3 mol per l, 4#: 4 mol per l, SIS-5#: 5 mol per l, SIS-6#: 6 mol per l and SIS-7#: 7 mol per l, respectively. The mixtures were stirred for 24 h at room temperature. All the experiments were performed in an argon-filled glove box. The carbon/sulphur composite is prepared by a melt-diffusion method. According to Ji et al.10, CMK-3 (Nanjing XF-nano, China) or Ketjenblack and sulphur were ground together, sealed in a glass tube containing argon, and heated at 155 °C for 24 h. (see Supplementary Figs S11–S13 and Supplementary Table S3). Characterizations. The conductivity of electrolyte system was measured with an impedance analyser (Zahner Zennium, Germany) over a temperature range of 20 to 60 °C in a thermostated container. The lithium-ion transference numbers were obtained by combining alternating-current (AC) impedance and direct-cur- rent (DC) polarization measurements using a symmetric Li/electrolyte/Li cell. First, AC impedance test was performed to obtain a total resistance Rcell. Then DC polarization was carried out to obtain a stable current IDC. The lithium-ion transference number was calculated by the formulas (tLi þ 1⁄4 Rcell/RDC and RDC 1⁄4 VDC/IDC). DSC measurements were performed with a NETZSCH DSC 200F3 by sealing B10 mg of the sample in an aluminium pan. The pan and the electrolyte were first cooled to about 150 °C with liquid nitrogen and then heated to 50 °C at a heating rate of 10 °C min 1 under nitrogen flow. The viscosity (Z) of the electrolytes was measured on a programmable viscometer (Brookfield, DV-iii þ ) at 25 °C in a homemade dry chamber (H2O o10 p.p.m.), and the temperature was accurately controlled to within ±0.1 °C using a Brookfield TC-502 oil bath. A SEM microscopy (Hitachi S-4800) equipped with a vacuum transfer box was used to study the morphology of the samples. The samples of lithium metal anode were stored in vacuum transfer box in an argon-filled glove box and transferred into SEM chamber without exposure to air. X-ray photoelectron spectroscopy analyses were performed in ESCALAB 250 using a monochromatized Al Ka source and equipped with an Ar þ ion sputtering 6 NATURE COMMUNICATIONS | 4:1481 | DOI: 10.1038/ncomms2513 | www.nature.com/naturecommunications & 2013 Macmillan Publishers Limited. All rights reserved. 4# Overpotential dissolution SIS-7# 0.06 24:42:39 74:22:14 24:52:41 Time (h) 49:58:41 Current (mA) Voltage (V) Current (mA) Voltage (V) Current (mA) Voltage (V)PDF Image | Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries
PDF Search Title:
Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteriesOriginal File Name Searched:
ncomms2513.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |