
PDF Publication Title:
Text from PDF Page: 007
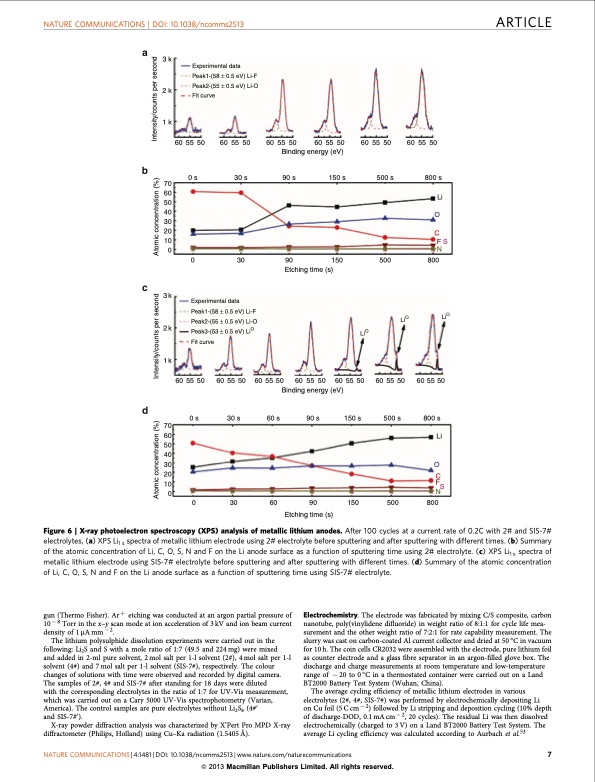
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms2513 ARTICLE 3k 2k 1k 70 60 50 40 30 20 10 3k 2k 1k 70 60 50 40 30 20 10 0 Experimental data Peak1-(58 ± 0.5 eV) Li-F Peak2-(55 ± 0.5 eV) Li-O Fit curve 60 55 50 0 s 60 55 50 30 s 60 55 50 Binding energy (eV) 60 55 50 500 s 60 55 50 800 s Li O C 90 s 150 s FS 0N 60 55 50 0 30 90 150 500 800 Etching time (s) 605550 0 s 605550 30 s 605550 Binding energy (eV) Experimental data Peak1-(58 ± 0.5 eV) Li-F Peak2-(55 ± 0.5 eV) Li-O Peak3-(53 ± 0.5 eV) LiO Fit curve LiO 150 s LiO 605550 500 s LiO 605550 800 s Li O C F 60 s 90 s 60 55 50 605550 0 30 60 90 150 500 800 Etching time (s) Figure 6 | X-ray photoelectron spectroscopy (XPS) analysis of metallic lithium anodes. After 100 cycles at a current rate of 0.2C with 2# and SIS-7# electrolytes, (a) XPS Li1 s spectra of metallic lithium electrode using 2# electrolyte before sputtering and after sputtering with different times. (b) Summary of the atomic concentration of Li, C, O, S, N and F on the Li anode surface as a function of sputtering time using 2# electrolyte. (c) XPS Li1 s spectra of metallic lithium electrode using SIS-7# electrolyte before sputtering and after sputtering with different times. (d) Summary of the atomic concentration of Li, C, O, S, N and F on the Li anode surface as a function of sputtering time using SIS-7# electrolyte. gun (Thermo Fisher). Arþ etching was conducted at an argon partial pressure of 10 8 Torr in the x–y scan mode at ion acceleration of 3 kV and ion beam current density of 1mAmm2. The lithium polysulphide dissolution experiments were carried out in the following: Li2S and S with a mole ratio of 1:7 (49.5 and 224 mg) were mixed and added in 2-ml pure solvent, 2 mol salt per 1-l solvent (2#), 4 mol salt per 1-l solvent (4#) and 7 mol salt per 1-l solvent (SIS-7#), respectively. The colour changes of solutions with time were observed and recorded by digital camera. The samples of 2#, 4# and SIS-7# after standing for 18 days were diluted with the corresponding electrolytes in the ratio of 1:7 for UV-Vis measurement, which was carried out on a Cary 5000 UV-Vis spectrophotometry (Varian, America). The control samples are pure electrolytes without Li2S8 (4#0 and SIS-7#0). X-ray powder diffraction analysis was characterized by X’Pert Pro MPD X-ray diffractometer (Philips, Holland) using Cu–Ka radiation (1.5405 Å). Electrochemistry. The electrode was fabricated by mixing C/S composite, carbon nanotube, poly(vinylidene difluoride) in weight ratio of 8:1:1 for cycle life mea- surement and the other weight ratio of 7:2:1 for rate capability measurement. The slurry was cast on carbon-coated Al current collector and dried at 50 °C in vacuum for 10 h. The coin cells CR2032 were assembled with the electrode, pure lithium foil as counter electrode and a glass fibre separator in an argon-filled glove box. The discharge and charge measurements at room temperature and low-temperature range of 20 to 0 °C in a thermostated container were carried out on a Land BT2000 Battery Test System (Wuhan, China). The average cycling efficiency of metallic lithium electrodes in various electrolytes (2#, 4#, SIS-7#) was performed by electrochemically depositing Li on Cu foil (5 C cm 2) followed by Li stripping and deposition cycling (10% depth of discharge-DOD, 0.1mAcm2, 20 cycles). The residual Li was then dissolved electrochemically (charged to 3 V) on a Land BT2000 Battery Test System. The average Li cycling efficiency was calculated according to Aurbach et al.53 NATURE COMMUNICATIONS | 4:1481 | DOI: 10.1038/ncomms2513 | www.nature.com/naturecommunications 7 & 2013 Macmillan Publishers Limited. All rights reserved. N S Atomic concentration (%) Intensity/counts per second Atomic concentration (%) Intensity/counts per secondPDF Image | Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries
PDF Search Title:
Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteriesOriginal File Name Searched:
ncomms2513.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |