
PDF Publication Title:
Text from PDF Page: 005
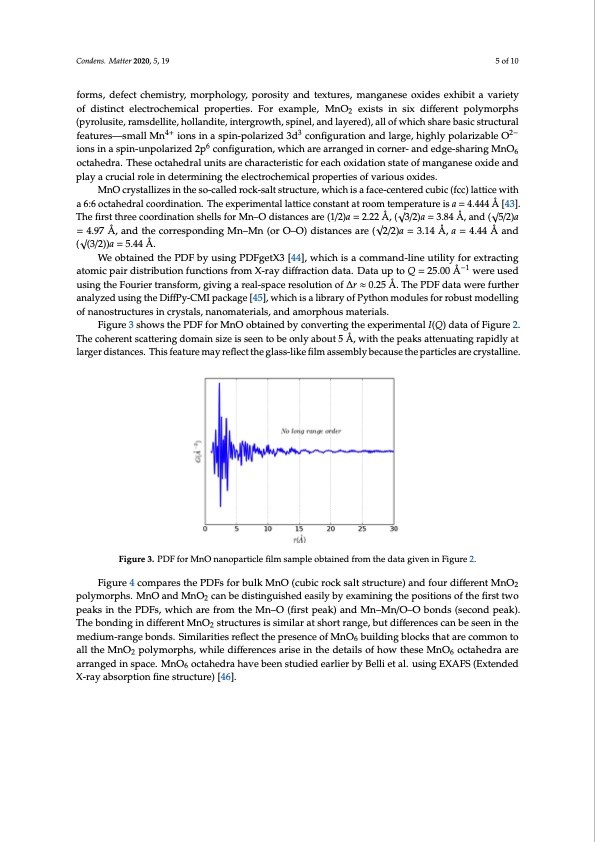
Condens. Matter 2019, 4, x FOR PEER REVIEW 5 of 10 3.2. An Illustrative Analysis of the MnO Data Condens. Matter 2020, 5, 19 5 of 10 Manganese can exist in the form of a variety of stable oxides (MnO, Mn3O4, Mn2O3, MnO2) [41,42], which crystallize in different types of structures. Associated with this wide diversity of crystal forms, defect chemistry, morphology, porosity and textures, manganese oxides exhibit a variety of forms, defect chemistry, morphology, porosity and textures, manganese oxides exhibit a variety distinct electrochemical properties. For example, MnO2 exists in six different polymorphs (pyrolusite, of distinct electrochemical properties. For example, MnO2 exists in six different polymorphs ramsdellite, hollandite, intergrowth, spinel, and layered), all of which share basic structural (pyrolusite, ramsdellite, hollandite, intergrowth, spinel, and layered), all of which share basic structural 4+ 3 2- features—small Mn4+ions in a spin-polarized 3d co3nfiguration and large, highly polarizable O ions2− features—small Mn ions in a spin-polarized 3d configuration and large, highly polarizable O in a spin-unpolarized 2p6 co6nfiguration, which are arranged in corner- and edge-sharing MnO6 ionsinaspin-unpolarized2p configuration,whicharearrangedincorner-andedge-sharingMnO6 octahedra. These octahedral units are characteristic for each oxidation state of manganese oxide and octahedra. These octahedral units are characteristic for each oxidation state of manganese oxide and play a crucial role in determining the electrochemical properties of various oxides. play a crucial role in determining the electrochemical properties of various oxides. MnO crystallizes in the so-called rock-salt structure, which is a face-centered cubic (fcc) lattice MnO crystallizes in the so-called rock-salt structure, which is a face-centered cubic (fcc) lattice with with a 6:6 octahedral coordination. The experimental lattice constant at room temperature is a = 4.444 a 6:6 octahedral coordination. The experimental lattice constant at room temperature is a = 4.444 Å [43]. Å [43]. The first three coordination shells for Mn–O distances are (1/2)a = 2.√22 Å, (√3/2)a = 3.84 Å,√and The first three coordination shells for Mn–O distances are (1/2)a = 2.22 Å, ( 3/2)a = 3.84 Å, and ( 5/2)a (√5/2)a = 4.97 Å, and the corresponding Mn–Mn (or O–O) distances√are (√2/2)a = 3.14 Å, a = 4.44 Å = 4.97 Å, and the corresponding Mn–Mn (or O–O) distances are ( 2/2)a = 3.14 Å, a = 4.44 Å and √and (√(3/2))a = 5.44 Å. ( (3/2))a = 5.44 Å. We obtained the PDF by using PDFgetX3 [44], which is a command-line utility for extracting We obtained the PDF by using PDFgetX3 [44], which is a command-line utility for extracting atomic pair distribution functions from X-ray diffraction data. Data up to Q = 25.00 Å–1 were used atomic pair distribution functions from X-ray diffraction data. Data up to Q = 25.00 Å−1 were used using the Fourier transform, giving a real-space resolution of Δr ≈ 0.25 Å. The PDF data were further using the Fourier transform, giving a real-space resolution of ∆r ≈ 0.25 Å. The PDF data were further analyzed using the DiffPy-CMI package [45], which is a library of Python modules for robust analyzed using the DiffPy-CMI package [45], which is a library of Python modules for robust modelling modelling of nanostructures in crystals, nanomaterials, and amorphous materials. of nanostructures in crystals, nanomaterials, and amorphous materials. Figure 3 shows the PDF for MnO obtained by converting the experimental I(Q) data of Figure 2. Figure 3 shows the PDF for MnO obtained by converting the experimental I(Q) data of Figure 2. The coherent scattering domain size is seen to be only about 5 Å, with the peaks attenuating rapidly The coherent scattering domain size is seen to be only about 5 Å, with the peaks attenuating rapidly at at larger distances. This feature may reflect the glass-like film assembly because the particles are larger distances. This feature may reflect the glass-like film assembly because the particles are crystalline. crystalline. Figure 3. PDF for MnO nanoparticle film sample obtained from the data given in Figure 2. Figure 3. PDF for MnO nanoparticle film sample obtained from the data given in Figure 2. Figure 4 compares the PDFs for bulk MnO (cubic rock salt structure) and four different MnO2 Figure 4 compares the PDFs for bulk MnO (cubic rock salt structure) and four different MnO2 polymorphs. MnO and MnO2 can be distinguished easily by examining the positions of the first two polymorphs. MnO and MnO2 can be distinguished easily by examining the positions of the first two peaks in the PDFs, which are from the Mn–O (first peak) and Mn–Mn/O–O bonds (second peak). The peaks in the PDFs, which are from the Mn–O (first peak) and Mn–Mn/O–O bonds (second peak). bonding in different MnO2 structures is similar at short range, but differences can be seen in the The bonding in different MnO2 structures is similar at short range, but differences can be seen in the medium-range bonds. Similarities reflect the presence of MnO6 building blocks that are common to medium-range bonds. Similarities reflect the presence of MnO6 building blocks that are common to all the MnO2 polymorphs, while differences arise in the details of how these MnO6 octahedra are all the MnO2 polymorphs, while differences arise in the details of how these MnO6 octahedra are arranged in space. MnO6 octahedra have been studied earlier by Belli et al. using EXAFS (Extended arranged in space. MnO6 octahedra have been studied earlier by Belli et al. using EXAFS (Extended X-ray absorption fine structure) [46]. X-ray absorption fine structure) [46].PDF Image | Structure of Manganese Oxide Nanoparticles Extracted via Pair Distribution Functions
PDF Search Title:
Structure of Manganese Oxide Nanoparticles Extracted via Pair Distribution FunctionsOriginal File Name Searched:
condensedmatter-05-00019-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |