
PDF Publication Title:
Text from PDF Page: 006
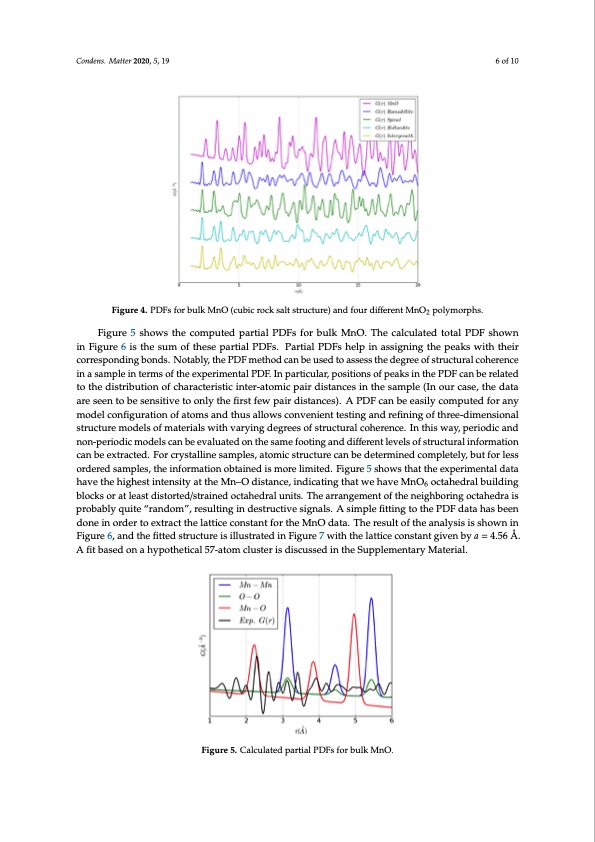
Condens. Matter 2019, 4, x FOR PEER REVIEW Condens. Matter 2020, 5, 19 6 of 10 Condens. Matter 2019, 4, x FOR PEER REVIEW 6 of 10 Figure 5 shows the computed partial PDFs for bulk MnO. The calculated total PDF shown in Figure 5 shows the computed partial PDFs for bulk MnO. The calculated total PDF shown Figure 5 shows the computed partial PDFs for bulk MnO. The calculated total PDF shown in Figure 6 is the sum of these partial PDFs. Partial PDFs help in assigning the peaks with their in Figure 6 is the sum of these partial PDFs. Partial PDFs help in assigning the peaks with their Figure 6 is the sum of these partial PDFs. Partial PDFs help in assigning the peaks with their corresponding bonds. Notably, the PDF method can be used to assess the degree of structural corresponding bonds. Notably, the PDF method can be used to assess the degree of structural coherence 6 of 10 Figure 4. PDFs for bulk MnO (cubic rock salt structure) and four different MnO2 polymorphs. Figure4.PDFsforbulkMnO(cubicrocksaltstructure)andfourdifferentMnO polymorphs. Figure 4. PDFs for bulk MnO (cubic rock salt structure) and four different MnO2 poly2 morphs. corresponding bonds. Notably, the PDF method can be used to assess the degree of structural coherence in a sample in terms of the experimental PDF. In particular, positions of peaks in the PDF in a sample in terms of the experimental PDF. In particular, positions of peaks in the PDF can be related coherence in a sample in terms of the experimental PDF. In particular, positions of peaks in the PDF can be related to the distribution of characteristic inter-atomic pair distances in the sample (In our to the distribution of characteristic inter-atomic pair distances in the sample (In our case, the data can be related to the distribution of characteristic inter-atomic pair distances in the sample (In our case, the data are seen to be sensitive to only the first few pair distances). A PDF can be easily acreassee,etnhetodbaetaseanresitsieveentotoobnelystehnesifitirvset fteowonplayirthdeisftiarnstcefse)w. ApaPirDdFisctaancbes)e.aAsilyPDcoFmcapnutbeed efoasrilayny computed for any model configuration of atoms and thus allows convenient testing and refining of mcomdepluctoendfifgourrantyiomnodfealtocmonsfiagnudratthiounsoaflloatwomscsoannvdenthieunstatlelostwinsgcoanvderneifientintegsotifntgharened-dreimfineinsgionfal three-dimensional structure models of materials with varying degrees of structural coherence. In this strhurceteu-dreimeondsieolnsaolfsmtruatcetruiraelsmwoidthelsvaorfyminagtedrieaglsrewesithofvsatryuicntgurdaelgcroeheseroefnscteru. cIntutrhailscwohaeyr,epnecrei.oIdnicthaisnd way, periodic and non-periodic models can be evaluated on the same footing and different levels of nwona-yp,epreioridoidcimc aonddelsnocann-pbeerieovdailcumatoedeolsnctahne sbaemeveafoluoattinedg aondthdeiffsearmenetfloeovteinlsgoafnsdtrudciftfueraelnitnlfeovremlsatoifon structural information can be extracted. For crystalline samples, atomic structure can be determined castnrubcetuerxatrlainctfeodrm. Faotirocnrycasntablleinexstraamctpelde.sF,oatrocmryisctastlrliuncetusaremcpalnesb,eatdoemteicrmstirnuecdtucroemcapnlebtelyd,ebteurtmfoinreldess completely, but for less ordered samples, the information obtained is more limited. Figure 5 shows ocrodmerpedletsealmy,pbluest,ftohreleinsfsoorrmdaetrieodnsoabmtapilneesd, tihseminofroerlmimatitioedn.oFbitgauinred5isshmoworsethliamtittheed.exFpigeurirme 5ensthaolwdasta that the experimental data have the highest intensity at the Mn–O distance, indicating that we have that the experimental data have the highest intensity at the Mn–O distance, indicating that we have have the highest intensity at the Mn–O distance, indicating that we have MnO6 octahedral building MnO6 octahedral building blocks or at least distorted/strained octahedral units. The arrangement of MnO6 octahedral building blocks or at least distorted/strained octahedral units. The arrangement of blocks or at least distorted/strained octahedral units. The arrangement of the neighboring octahedra is the neighboring octahedra is probably quite “random”, resulting in destructive signals. A simple the neighboring octahedra is probably quite “random”, resulting in destructive signals. A simple probably quite “random”, resulting in destructive signals. A simple fitting to the PDF data has been fitting to the PDF data has been done in order to extract the lattice constant for the MnO data. The fitting to the PDF data has been done in order to extract the lattice constant for the MnO data. The done in order to extract the lattice constant for the MnO data. The result of the analysis is shown in result of the analysis is shown in Figure 6, and the fitted structure is illustrated in Figure 7 with the result of the analysis is shown in Figure 6, and the fitted structure is illustrated in Figure 7 with the Figure 6, and the fitted structure is illustrated in Figure 7 with the lattice constant given by a = 4.56 Å. lattice constant given by a = 4.56 Å. A fit based on a hypothetical 57-atom cluster is discussed in the lattice constant given by a = 4.56 Å. A fit based on a hypothetical 57-atom cluster is discussed in the A fit based on a hypothetical 57-atom cluster is discussed in the Supplementary Material. Supplementary Material. Supplementary Material. Figure 5. Calculated partial PDFs for bulk MnO. Figure 5. Calculated partial PDFs for bulk MnO. Figure 5. Calculated partial PDFs for bulk MnO.PDF Image | Structure of Manganese Oxide Nanoparticles Extracted via Pair Distribution Functions
PDF Search Title:
Structure of Manganese Oxide Nanoparticles Extracted via Pair Distribution FunctionsOriginal File Name Searched:
condensedmatter-05-00019-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |