
PDF Publication Title:
Text from PDF Page: 005
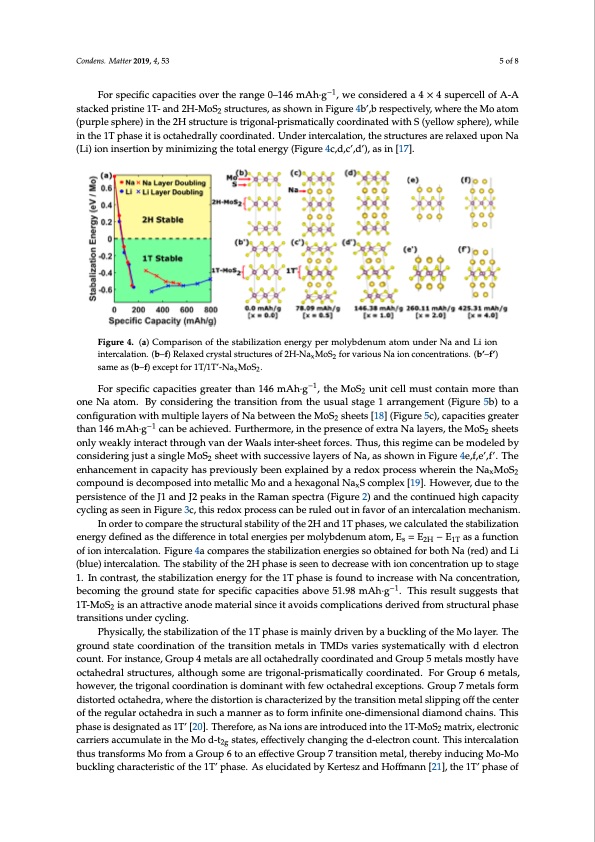
Condens. Matter 2019, 4, 53 5 of 8 For specific capacities over the range 0–146 mAh·g−1, we considered a 4 × 4 supercell of A-A stacked pristine 1T- and 2H-MoS2 structures, as shown in Figure 4b’,b respectively, where the Mo atom (purple sphere) in the 2H structure is trigonal-prismatically coordinated with S (yellow sphere), while in the 1T phase it is octahedrally coordinated. Under intercalation, the structures are relaxed upon Na (Li) ion insertion by minimizing the total energy (Figure 4c,d,c’,d’), as in [17]. Condens. Matter 2019, 4, x FOR PEER REVIEW 6 of 10 Figure 4. (a) Comparison of the stabilization energy per molybdenum atom under Na and Figure 4. (a) Comparison of the stabilization energy per molybdenum atom under Na and Li ion iLniteirocnalaitniotenr.c(abl–aft)ioRnel.ax(bed)–c(rfy)sRtaellsatrxuecdtucrersyostfa2lHs-tNruacMtuorSesforfv2aHri-oNusaNxMaoioSn2cfonrcevnatriaotiuosnsN. (ab’i–ofn’) x2 scaomneceanst(rba–tfio)enxsc.ep(tbfo’)r–1(fT’/)1sTa’-mNeaaMso(bS).–(f)exceptfor1T/1T’-NaxMoS2. x2 −1 For specific capacities greater than 146 mAh∙g , the MoS2 unit cell must contain more than one For specific capacities greater than 146 mAh·g-1 one Na atom. By considering the transition from the usual stage 1 arrangement (Figure 5b) to a Na atom. By considering the transition from the usual stage 1 arrangement (Figure 5b) to a configurationwithmultiplelayersofNabetweentheMoS sheets[18](Figure5c),capacitiesgreater 2 configuration with multiple layers of Na between the MoS2 sheets [18] (Figure 5c), capacities greater −1 than 146 mAh·g −1 can be achieved. Furthermore, in the presence of extra Na layers, the MoS than 146 mAh∙g can be achieved. Furthermore, in the presence of extra Na layers, the MoS2 sheets sheets only weakly interact through van der Waals inter-sheet forces. Thus, this regime can be modeled by only weakly interact through van der Waals inter-sheet forces. Thus, this regime can be modeled by consideringjustasingleMoS sheetwithsuccessivelayersofNa,asshowninFigure4e,f,e’,f’.The 2 considering just a single MoS2 sheet with successive layers of Na, as shown in Figure 4e,f and 4e’,f’. enhancement in capacity has previously been explained by a redox process wherein the NaxMoS2 The enhancement in capacity has previously been explained by a redox process wherein the compound is decomposed into metallic Mo and a hexagonal NaxS complex [19]. However, due to the NaxMoS2 compound is decomposed into metallic Mo and a hexagonal NaxS complex [19]. However, persistence of the J1 and J2 peaks in the Raman spectra (Figure 2) and the continued high capacity due to the persistence of the J1 and J2 peaks in the Raman spectra (Figure 2) and the continued high cycling as seen in Figure 3c, this redox process can be ruled out in favor of an intercalation mechanism. capacity cycling as seen in Figure 3c, this redox process can be ruled out in favor of an intercalation In order to compare the structural stability of the 2H and 1T phases, we calculated the stabilization mechanism. energy defined as the difference in total energies per molybdenum atom, Es = E2H − E1T as a function of ion intercalation. Figure 4a compares the stabilization energies so obtained for both Na (red) and Li (blue) intercalation. The stability of the 2H phase is seen to decrease with ion concentration up to stage 1. In contrast, the stabilization energy for the 1T phase is found to increase with Na concentration, becoming the ground state for specific capacities above 51.98 mAh·g−1. This result suggests that 1T-MoS2 is an attractive anode material since it avoids complications derived from structural phase transitions under cycling. Physically, the stabilization of the 1T phase is mainly driven by a buckling of the Mo layer. The ground state coordination of the transition metals in TMDs varies systematically with d electron count. For instance, Group 4 metals are all octahedrally coordinated and Group 5 metals mostly have octahedral structures, although some are trigonal-prismatically coordinated. For Group 6 metals, however, the trigonal coordination is dominant with few octahedral exceptions. Group 7 metals form distorted octahedra, where the distortion is characterized by the transition metal slipping off the center of the regular octahedra in such a manner as to form infinite one-dimensional diamond chains. This phase is designated as 1T’ [20]. Therefore, as Na ions are introduced into the 1T-MoS2 matrix, electronic carriers accumulate in the Mo d-t2g states, effectively changing the d-electron count. This intercalation thus transforms Mo from a Group 6 to an effective Group 7 transition metal, thereby inducing Mo-Mo buckling characteristic of the 1T’ phase. As elucidated by Kertesz and Hoffmann [21], the 1T’ phase of , the MoS 2 unit cell must contain more than 2PDF Image | Understanding Phase Stability of Metallic 1T-MoS2 Anodes for Sodium-Ion Batteries
PDF Search Title:
Understanding Phase Stability of Metallic 1T-MoS2 Anodes for Sodium-Ion BatteriesOriginal File Name Searched:
condensedmatter-04-00053.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |