
PDF Publication Title:
Text from PDF Page: 007
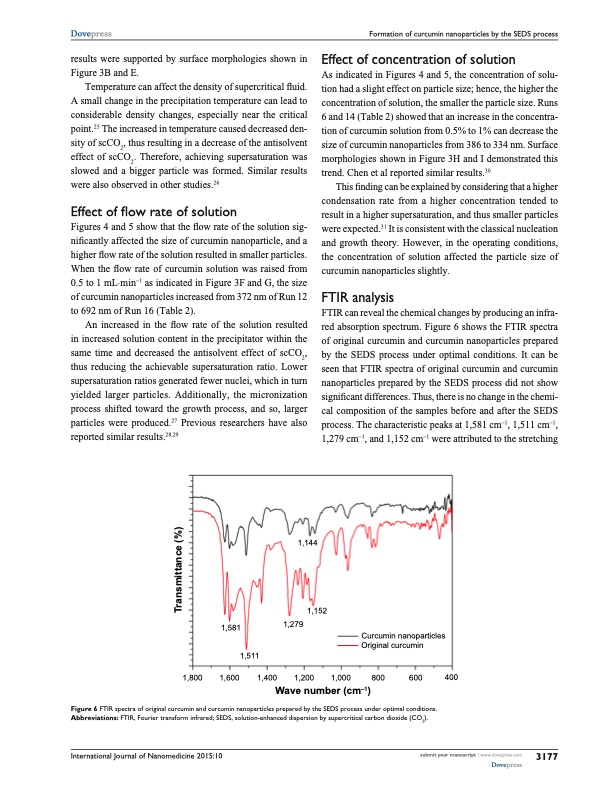
Dovepress Formation of curcumin nanoparticles by the seDs process results were supported by surface morphologies shown in Figure 3B and E. Temperature can affect the density of supercritical fluid. A small change in the precipitation temperature can lead to considerable density changes, especially near the critical point.25 The increased in temperature caused decreased den- sity of scCO2, thus resulting in a decrease of the antisolvent effect of scCO2. Therefore, achieving supersaturation was slowed and a bigger particle was formed. Similar results were also observed in other studies.26 Effect of flow rate of solution Figures 4 and 5 show that the flow rate of the solution sig- nificantly affected the size of curcumin nanoparticle, and a higher flow rate of the solution resulted in smaller particles. When the flow rate of curcumin solution was raised from 0.5 to 1 mL⋅min-1 as indicated in Figure 3F and G, the size of curcumin nanoparticles increased from 372 nm of Run 12 to 692 nm of Run 16 (Table 2). An increased in the flow rate of the solution resulted in increased solution content in the precipitator within the same time and decreased the antisolvent effect of scCO2, thus reducing the achievable supersaturation ratio. Lower supersaturation ratios generated fewer nuclei, which in turn yielded larger particles. Additionally, the micronization process shifted toward the growth process, and so, larger particles were produced.27 Previous researchers have also reported similar results.28,29 effect of concentration of solution As indicated in Figures 4 and 5, the concentration of solu- tion had a slight effect on particle size; hence, the higher the concentration of solution, the smaller the particle size. Runs 6 and 14 (Table 2) showed that an increase in the concentra- tion of curcumin solution from 0.5% to 1% can decrease the size of curcumin nanoparticles from 386 to 334 nm. Surface morphologies shown in Figure 3H and I demonstrated this trend. Chen et al reported similar results.30 This finding can be explained by considering that a higher condensation rate from a higher concentration tended to result in a higher supersaturation, and thus smaller particles were expected.31 It is consistent with the classical nucleation and growth theory. However, in the operating conditions, the concentration of solution affected the particle size of curcumin nanoparticles slightly. FTIr analysis FTIR can reveal the chemical changes by producing an infra- red absorption spectrum. Figure 6 shows the FTIR spectra of original curcumin and curcumin nanoparticles prepared by the SEDS process under optimal conditions. It can be seen that FTIR spectra of original curcumin and curcumin nanoparticles prepared by the SEDS process did not show significant differences. Thus, there is no change in the chemi- cal composition of the samples before and after the SEDS process. The characteristic peaks at 1,581 cm-1, 1,511 cm-1, 1,279 cm-1, and 1,152 cm-1 were attributed to the stretching ����� ����� ����� ����� ����� ����� ����� ����� ����� ����� ��� ��� ��� ���� ������ ������ Figure 6 FTIr spectra of original curcumin and curcumin nanoparticles prepared by the seDs process under optimal conditions. Abbreviations: FTIr, Fourier transform infrared; seDs, solution-enhanced dispersion by supercritical carbon dioxide (cO2). �������� ������������� �������� �������� International Journal of Nanomedicine 2015:10 submit your manuscript | www.dovepress.com Dovepress 3177 ������������� ���PDF Image | curcumin nanoparticles via dispersion by supercritical cO2
PDF Search Title:
curcumin nanoparticles via dispersion by supercritical cO2Original File Name Searched:
cucumin-nanoparticles-co2.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |