
PDF Publication Title:
Text from PDF Page: 002
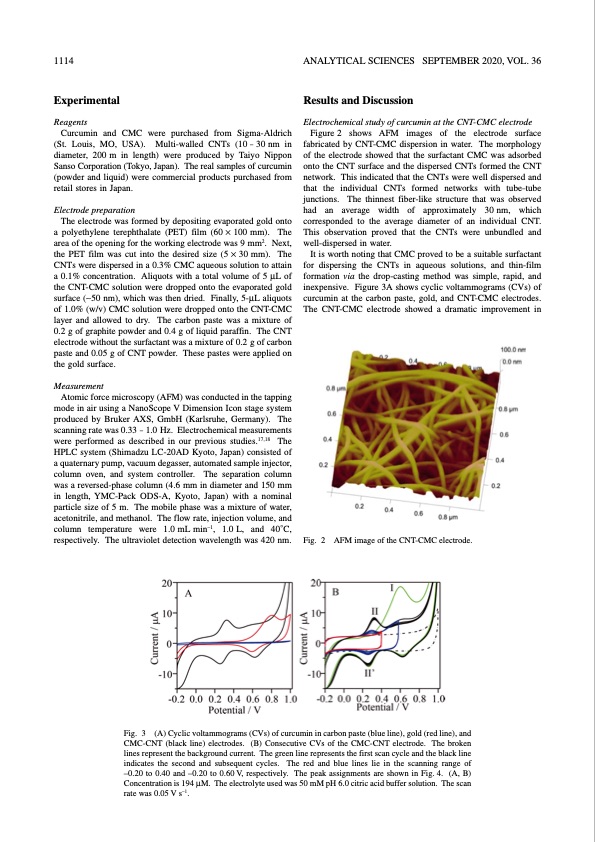
1114 ANALYTICAL SCIENCES SEPTEMBER 2020, VOL. 36 Experimental Reagents Curcumin and CMC were purchased from Sigma-Aldrich (St. Louis, MO, USA). Multi-walled CNTs (10 – 30 nm in diameter, 200 m in length) were produced by Taiyo Nippon Sanso Corporation (Tokyo, Japan). The real samples of curcumin (powder and liquid) were commercial products purchased from retail stores in Japan. Electrode preparation The electrode was formed by depositing evaporated gold onto a polyethylene terephthalate (PET) film (60 × 100 mm). The area of the opening for the working electrode was 9 mm2. Next, the PET film was cut into the desired size (5 × 30 mm). The CNTs were dispersed in a 0.3% CMC aqueous solution to attain a 0.1% concentration. Aliquots with a total volume of 5 μL of the CNT-CMC solution were dropped onto the evaporated gold surface (∼50 nm), which was then dried. Finally, 5-μL aliquots of 1.0% (w/v) CMC solution were dropped onto the CNT-CMC layer and allowed to dry. The carbon paste was a mixture of 0.2 g of graphite powder and 0.4 g of liquid paraffin. The CNT electrode without the surfactant was a mixture of 0.2 g of carbon paste and 0.05 g of CNT powder. These pastes were applied on the gold surface. Measurement Atomic force microscopy (AFM) was conducted in the tapping mode in air using a NanoScope V Dimension Icon stage system produced by Bruker AXS, GmbH (Karlsruhe, Germany). The scanning rate was 0.33 – 1.0 Hz. Electrochemical measurements were performed as described in our previous studies.17,18 The HPLC system (Shimadzu LC-20AD Kyoto, Japan) consisted of a quaternary pump, vacuum degasser, automated sample injector, column oven, and system controller. The separation column was a reversed-phase column (4.6 mm in diameter and 150 mm in length, YMC-Pack ODS-A, Kyoto, Japan) with a nominal particle size of 5 m. The mobile phase was a mixture of water, acetonitrile, and methanol. The flow rate, injection volume, and column temperature were 1.0mLmin–1, 1.0L, and 40°C, respectively. The ultraviolet detection wavelength was 420 nm. Results and Discussion Electrochemical study of curcumin at the CNT-CMC electrode Figure 2 shows AFM images of the electrode surface fabricated by CNT-CMC dispersion in water. The morphology of the electrode showed that the surfactant CMC was adsorbed onto the CNT surface and the dispersed CNTs formed the CNT network. This indicated that the CNTs were well dispersed and that the individual CNTs formed networks with tube–tube junctions. The thinnest fiber-like structure that was observed had an average width of approximately 30nm, which corresponded to the average diameter of an individual CNT. This observation proved that the CNTs were unbundled and well-dispersed in water. It is worth noting that CMC proved to be a suitable surfactant for dispersing the CNTs in aqueous solutions, and thin-film formation via the drop-casting method was simple, rapid, and inexpensive. Figure 3A shows cyclic voltammograms (CVs) of curcumin at the carbon paste, gold, and CNT-CMC electrodes. The CNT-CMC electrode showed a dramatic improvement in Fig. 2 AFM image of the CNT-CMC electrode. Fig. 3 (A) Cyclic voltammograms (CVs) of curcumin in carbon paste (blue line), gold (red line), and CMC-CNT (black line) electrodes. (B) Consecutive CVs of the CMC-CNT electrode. The broken lines represent the background current. The green line represents the first scan cycle and the black line indicates the second and subsequent cycles. The red and blue lines lie in the scanning range of –0.20 to 0.40 and –0.20 to 0.60 V, respectively. The peak assignments are shown in Fig. 4. (A, B) Concentration is 194 μM. The electrolyte used was 50 mM pH 6.0 citric acid buffer solution. The scan rate was 0.05 V s–1.PDF Image | Electrochemical Detection of Curcumin
PDF Search Title:
Electrochemical Detection of CurcuminOriginal File Name Searched:
36_20P021.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |