
PDF Publication Title:
Text from PDF Page: 003
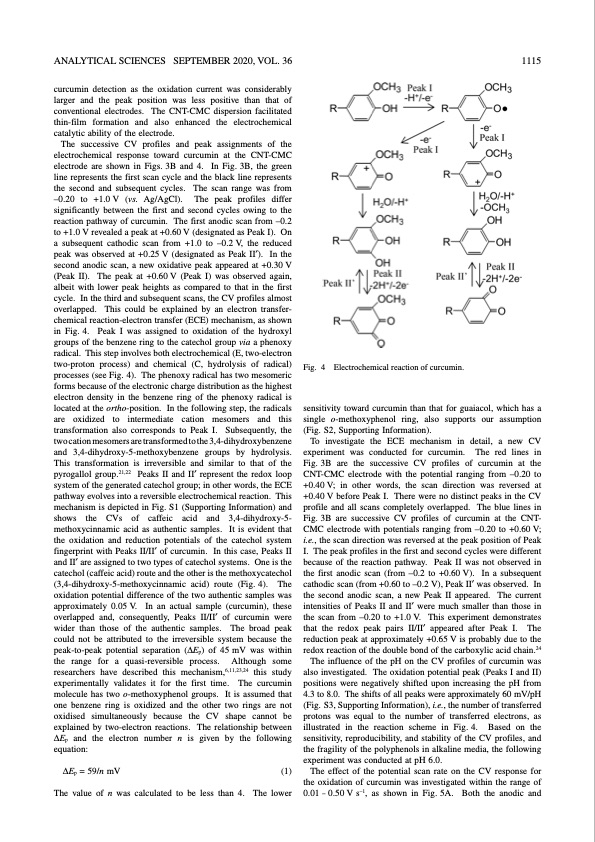
ANALYTICAL SCIENCES SEPTEMBER 2020, VOL. 36 1115 curcumin detection as the oxidation current was considerably larger and the peak position was less positive than that of conventional electrodes. The CNT-CMC dispersion facilitated thin-film formation and also enhanced the electrochemical catalytic ability of the electrode. The successive CV profiles and peak assignments of the electrochemical response toward curcumin at the CNT-CMC electrode are shown in Figs. 3B and 4. In Fig. 3B, the green line represents the first scan cycle and the black line represents the second and subsequent cycles. The scan range was from –0.20 to +1.0 V (vs. Ag/AgCl). The peak profiles differ significantly between the first and second cycles owing to the reaction pathway of curcumin. The first anodic scan from –0.2 to +1.0 V revealed a peak at +0.60 V (designated as Peak I). On a subsequent cathodic scan from +1.0 to –0.2 V, the reduced peak was observed at +0.25 V (designated as Peak II′). In the second anodic scan, a new oxidative peak appeared at +0.30 V (Peak II). The peak at +0.60 V (Peak I) was observed again, albeit with lower peak heights as compared to that in the first cycle. In the third and subsequent scans, the CV profiles almost overlapped. This could be explained by an electron transfer- chemical reaction-electron transfer (ECE) mechanism, as shown in Fig. 4. Peak I was assigned to oxidation of the hydroxyl groups of the benzene ring to the catechol group via a phenoxy radical. This step involves both electrochemical (E, two-electron two-proton process) and chemical (C, hydrolysis of radical) processes (see Fig. 4). The phenoxy radical has two mesomeric forms because of the electronic charge distribution as the highest electron density in the benzene ring of the phenoxy radical is located at the ortho-position. In the following step, the radicals are oxidized to intermediate cation mesomers and this transformation also corresponds to Peak I. Subsequently, the two cation mesomers are transformed to the 3,4-dihydroxybenzene and 3,4-dihydroxy-5-methoxybenzene groups by hydrolysis. This transformation is irreversible and similar to that of the pyrogallol group.21,22 Peaks II and II′ represent the redox loop system of the generated catechol group; in other words, the ECE pathway evolves into a reversible electrochemical reaction. This mechanism is depicted in Fig. S1 (Supporting Information) and shows the CVs of caffeic acid and 3,4-dihydroxy-5- methoxycinnamic acid as authentic samples. It is evident that the oxidation and reduction potentials of the catechol system fingerprint with Peaks II/II′ of curcumin. In this case, Peaks II and II′ are assigned to two types of catechol systems. One is the catechol (caffeic acid) route and the other is the methoxycatechol (3,4-dihydroxy-5-methoxycinnamic acid) route (Fig. 4). The oxidation potential difference of the two authentic samples was approximately 0.05 V. In an actual sample (curcumin), these overlapped and, consequently, Peaks II/II′ of curcumin were wider than those of the authentic samples. The broad peak could not be attributed to the irreversible system because the peak-to-peak potential separation (ΔEp) of 45 mV was within the range for a quasi-reversible process. Although some researchers have described this mechanism,6,11,23,24 this study experimentally validates it for the first time. The curcumin molecule has two o-methoxyphenol groups. It is assumed that one benzene ring is oxidized and the other two rings are not oxidised simultaneously because the CV shape cannot be explained by two-electron reactions. The relationship between ΔEp and the electron number n is given by the following equation: ΔEp = 59/n mV (1) The value of n was calculated to be less than 4. The lower Fig. 4 Electrochemical reaction of curcumin. sensitivity toward curcumin than that for guaiacol, which has a single o-methoxyphenol ring, also supports our assumption (Fig. S2, Supporting Information). To investigate the ECE mechanism in detail, a new CV experiment was conducted for curcumin. The red lines in Fig. 3B are the successive CV profiles of curcumin at the CNT-CMC electrode with the potential ranging from –0.20 to +0.40 V; in other words, the scan direction was reversed at +0.40 V before Peak I. There were no distinct peaks in the CV profile and all scans completely overlapped. The blue lines in Fig. 3B are successive CV profiles of curcumin at the CNT- CMC electrode with potentials ranging from –0.20 to +0.60 V; i.e., the scan direction was reversed at the peak position of Peak I. The peak profiles in the first and second cycles were different because of the reaction pathway. Peak II was not observed in the first anodic scan (from –0.2 to +0.60 V). In a subsequent cathodic scan (from +0.60 to –0.2 V), Peak II′ was observed. In the second anodic scan, a new Peak II appeared. The current intensities of Peaks II and II′ were much smaller than those in the scan from –0.20 to +1.0 V. This experiment demonstrates that the redox peak pairs II/II′ appeared after Peak I. The reduction peak at approximately +0.65 V is probably due to the redox reaction of the double bond of the carboxylic acid chain.24 The influence of the pH on the CV profiles of curcumin was also investigated. The oxidation potential peak (Peaks I and II) positions were negatively shifted upon increasing the pH from 4.3 to 8.0. The shifts of all peaks were approximately 60 mV/pH (Fig. S3, Supporting Information), i.e., the number of transferred protons was equal to the number of transferred electrons, as illustrated in the reaction scheme in Fig. 4. Based on the sensitivity, reproducibility, and stability of the CV profiles, and the fragility of the polyphenols in alkaline media, the following experiment was conducted at pH 6.0. The effect of the potential scan rate on the CV response for the oxidation of curcumin was investigated within the range of 0.01 – 0.50 V s–1, as shown in Fig. 5A. Both the anodic andPDF Image | Electrochemical Detection of Curcumin
PDF Search Title:
Electrochemical Detection of CurcuminOriginal File Name Searched:
36_20P021.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |