
PDF Publication Title:
Text from PDF Page: 002
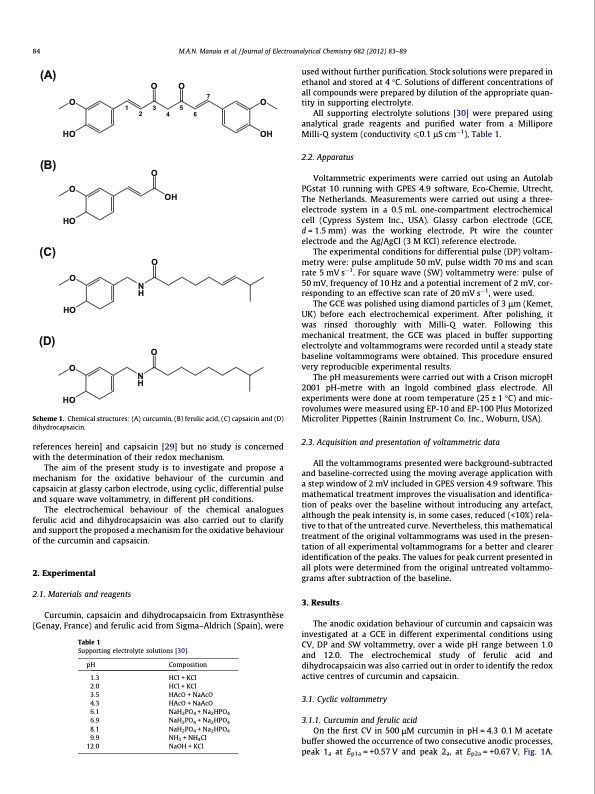
84 M.A.N. Manaia et al. / Journal of Electroanalytical Chemistry 682 (2012) 83–89 (A) OO O7O 135 HO (B) O HO (C) HO (D) O HO 246 O OH O O NH OH O NH Scheme 1. Chemical structures: (A) curcumin, (B) ferulic acid, (C) capsaicin and (D) dihydrocapsaicin. references herein] and capsaicin [29] but no study is concerned with the determination of their redox mechanism. The aim of the present study is to investigate and propose a mechanism for the oxidative behaviour of the curcumin and capsaicin at glassy carbon electrode, using cyclic, differential pulse and square wave voltammetry, in different pH conditions. The electrochemical behaviour of the chemical analogues ferulic acid and dihydrocapsaicin was also carried out to clarify and support the proposed a mechanism for the oxidative behaviour of the curcumin and capsaicin. 2. Experimental 2.1. Materials and reagents Curcumin, capsaicin and dihydrocapsaicin from Extrasynthèse (Genay, France) and ferulic acid from Sigma–Aldrich (Spain), were Table 1 Supporting electrolyte solutions [30]. pH Composition 1.3 HCl + KCl 2.0 HCl + KCl 3.5 HAcO + NaAcO 4.3 HAcO + NaAcO 6.1 NaH2PO4 + Na2HPO4 6.9 NaH2PO4 + Na2HPO4 8.1 NaH2PO4 + Na2HPO4 9.9 NH3 + NH4Cl 12.0 NaOH + KCl used without further purification. Stock solutions were prepared in ethanol and stored at 4 °C. Solutions of different concentrations of all compounds were prepared by dilution of the appropriate quan- tity in supporting electrolyte. All supporting electrolyte solutions [30] were prepared using analytical grade reagents and purified water from a Millipore Milli-Q system (conductivity 60.1 lS cm1), Table 1. 2.2. Apparatus Voltammetric experiments were carried out using an Autolab PGstat 10 running with GPES 4.9 software, Eco-Chemie, Utrecht, The Netherlands. Measurements were carried out using a three- electrode system in a 0.5 mL one-compartment electrochemical cell (Cypress System Inc., USA). Glassy carbon electrode (GCE, d = 1.5 mm) was the working electrode, Pt wire the counter electrode and the Ag/AgCl (3 M KCl) reference electrode. The experimental conditions for differential pulse (DP) voltam- metry were: pulse amplitude 50 mV, pulse width 70 ms and scan rate 5 mV s1. For square wave (SW) voltammetry were: pulse of 50 mV, frequency of 10 Hz and a potential increment of 2 mV, cor- responding to an effective scan rate of 20 mV s1, were used. The GCE was polished using diamond particles of 3 lm (Kemet, UK) before each electrochemical experiment. After polishing, it was rinsed thoroughly with Milli-Q water. Following this mechanical treatment, the GCE was placed in buffer supporting electrolyte and voltammograms were recorded until a steady state baseline voltammograms were obtained. This procedure ensured very reproducible experimental results. The pH measurements were carried out with a Crison micropH 2001 pH-metre with an Ingold combined glass electrode. All experiments were done at room temperature (25 ± 1 °C) and mic- rovolumes were measured using EP-10 and EP-100 Plus Motorized Microliter Pippettes (Rainin Instrument Co. Inc., Woburn, USA). 2.3. Acquisition and presentation of voltammetric data All the voltammograms presented were background-subtracted and baseline-corrected using the moving average application with a step window of 2 mV included in GPES version 4.9 software. This mathematical treatment improves the visualisation and identifica- tion of peaks over the baseline without introducing any artefact, although the peak intensity is, in some cases, reduced (<10%) rela- tive to that of the untreated curve. Nevertheless, this mathematical treatment of the original voltammograms was used in the presen- tation of all experimental voltammograms for a better and clearer identification of the peaks. The values for peak current presented in all plots were determined from the original untreated voltammo- grams after subtraction of the baseline. 3. Results The anodic oxidation behaviour of curcumin and capsaicin was investigated at a GCE in different experimental conditions using CV, DP and SW voltammetry, over a wide pH range between 1.0 and 12.0. The electrochemical study of ferulic acid and dihydrocapsaicin was also carried out in order to identify the redox active centres of curcumin and capsaicin. 3.1. Cyclic voltammetry 3.1.1. Curcumin and ferulic acid On the first CV in 500 lM curcumin in pH = 4.3 0.1 M acetate buffer showed the occurrence of two consecutive anodic processes, peak 1a at Ep1a = +0.57 V and peak 2a, at Ep2a = +0.67 V, Fig. 1A.PDF Image | Guaicolic spices curcumin and capsaicin electrochemical oxidation
PDF Search Title:
Guaicolic spices curcumin and capsaicin electrochemical oxidationOriginal File Name Searched:
170-curcumin-JEAC.PDFDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |