
PDF Publication Title:
Text from PDF Page: 003
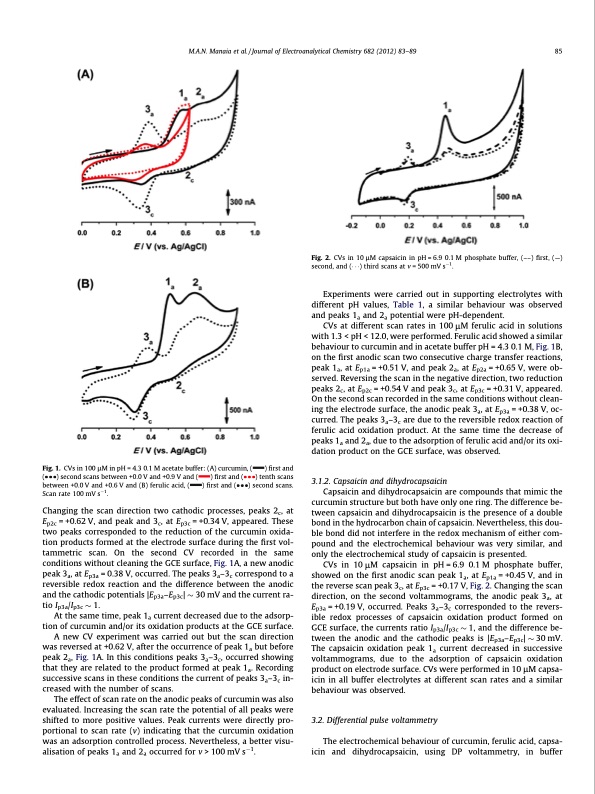
M.A.N. Manaia et al. / Journal of Electroanalytical Chemistry 682 (2012) 83–89 85 Fig. 1. CVs in 100 lM in pH = 4.3 0.1 M acetate buffer: (A) curcumin, ( ) first and ( ) second scans between +0.0 V and +0.9 V and ( ) first and ( ) tenth scans between +0.0 V and +0.6 V and (B) ferulic acid, ( ) first and ( ) second scans. Scan rate 100 mV s1. Fig. 2. CVs in 10 lM capsaicin in pH = 6.9 0.1 M phosphate buffer, (––) first, (—) second, and ( ) third scans at v = 500 mV s1. Changing the scan direction two cathodic processes, peaks 2c, at Ep2c = +0.62 V, and peak and 3c, at Ep3c = +0.34 V, appeared. These two peaks corresponded to the reduction of the curcumin oxida- tion products formed at the electrode surface during the first vol- tammetric scan. On the second CV recorded in the same conditions without cleaning the GCE surface, Fig. 1A, a new anodic peak 3a, at Ep3a = 0.38 V, occurred. The peaks 3a–3c correspond to a reversible redox reaction and the difference between the anodic and the cathodic potentials |Ep3a–Ep3c| 30 mV and the current ra- tio Ip3a/Ip3c 1. At the same time, peak 1a current decreased due to the adsorp- tion of curcumin and/or its oxidation products at the GCE surface. A new CV experiment was carried out but the scan direction was reversed at +0.62 V, after the occurrence of peak 1a but before peak 2a, Fig. 1A. In this conditions peaks 3a–3c, occurred showing that they are related to the product formed at peak 1a. Recording successive scans in these conditions the current of peaks 3a–3c in- creased with the number of scans. The effect of scan rate on the anodic peaks of curcumin was also evaluated. Increasing the scan rate the potential of all peaks were shifted to more positive values. Peak currents were directly pro- portional to scan rate (v) indicating that the curcumin oxidation was an adsorption controlled process. Nevertheless, a better visu- alisation of peaks 1a and 2a occurred for v > 100 mV s1. Experiments were carried out in supporting electrolytes with different pH values, Table 1, a similar behaviour was observed and peaks 1a and 2a potential were pH-dependent. CVs at different scan rates in 100 lM ferulic acid in solutions with 1.3 < pH < 12.0, were performed. Ferulic acid showed a similar behaviour to curcumin and in acetate buffer pH = 4.3 0.1 M, Fig. 1B, on the first anodic scan two consecutive charge transfer reactions, peak 1a, at Ep1a = +0.51 V, and peak 2a, at Ep2a = +0.65 V, were ob- served. Reversing the scan in the negative direction, two reduction peaks 2c, at Ep2c = +0.54 V and peak 3c, at Ep3c = +0.31 V, appeared. On the second scan recorded in the same conditions without clean- ing the electrode surface, the anodic peak 3a, at Ep3a = +0.38 V, oc- curred. The peaks 3a–3c are due to the reversible redox reaction of ferulic acid oxidation product. At the same time the decrease of peaks 1a and 2a, due to the adsorption of ferulic acid and/or its oxi- dation product on the GCE surface, was observed. 3.1.2. Capsaicin and dihydrocapsaicin Capsaicin and dihydrocapsaicin are compounds that mimic the curcumin structure but both have only one ring. The difference be- tween capsaicin and dihydrocapsaicin is the presence of a double bond in the hydrocarbon chain of capsaicin. Nevertheless, this dou- ble bond did not interfere in the redox mechanism of either com- pound and the electrochemical behaviour was very similar, and only the electrochemical study of capsaicin is presented. CVs in 10 lM capsaicin in pH = 6.9 0.1 M phosphate buffer, showed on the first anodic scan peak 1a, at Ep1a = +0.45 V, and in the reverse scan peak 3c, at Ep3c = +0.17 V, Fig. 2. Changing the scan direction, on the second voltammograms, the anodic peak 3a, at Ep3a = +0.19 V, occurred. Peaks 3a–3c corresponded to the revers- ible redox processes of capsaicin oxidation product formed on GCE surface, the currents ratio Ip3a/Ip3c 1, and the difference be- tween the anodic and the cathodic peaks is |Ep3a–Ep3c| 30 mV. The capsaicin oxidation peak 1a current decreased in successive voltammograms, due to the adsorption of capsaicin oxidation product on electrode surface. CVs were performed in 10 lM capsa- icin in all buffer electrolytes at different scan rates and a similar behaviour was observed. 3.2. Differential pulse voltammetry The electrochemical behaviour of curcumin, ferulic acid, capsa- icin and dihydrocapsaicin, using DP voltammetry, in bufferPDF Image | Guaicolic spices curcumin and capsaicin electrochemical oxidation
PDF Search Title:
Guaicolic spices curcumin and capsaicin electrochemical oxidationOriginal File Name Searched:
170-curcumin-JEAC.PDFDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |