
PDF Publication Title:
Text from PDF Page: 004
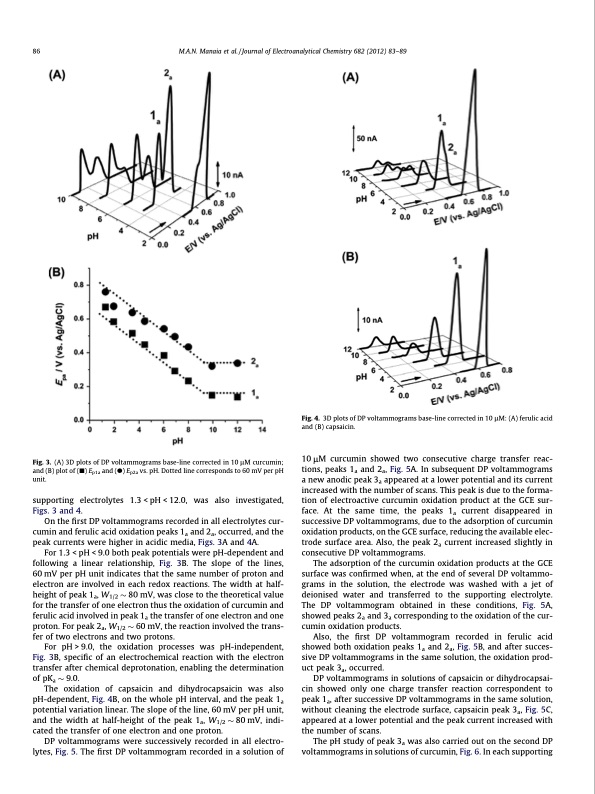
86 M.A.N. Manaia et al. / Journal of Electroanalytical Chemistry 682 (2012) 83–89 Fig. 4. 3D plots of DP voltammograms base-line corrected in 10 lM: (A) ferulic acid and (B) capsaicin. Fig. 3. (A) 3D plots of DP voltammograms base-line corrected in 10 lM curcumin; and (B) plot of (j) Ep1a and (d) Ep2a vs. pH. Dotted line corresponds to 60 mV per pH unit. supporting Figs. 3 and 4. electrolytes 1.3 < pH < 12.0, was also investigated, 10 lM curcumin showed two consecutive charge transfer reac- tions, peaks 1a and 2a, Fig. 5A. In subsequent DP voltammograms a new anodic peak 3a appeared at a lower potential and its current increased with the number of scans. This peak is due to the forma- tion of electroactive curcumin oxidation product at the GCE sur- face. At the same time, the peaks 1a current disappeared in successive DP voltammograms, due to the adsorption of curcumin oxidation products, on the GCE surface, reducing the available elec- trode surface area. Also, the peak 2a current increased slightly in consecutive DP voltammograms. The adsorption of the curcumin oxidation products at the GCE surface was confirmed when, at the end of several DP voltammo- grams in the solution, the electrode was washed with a jet of deionised water and transferred to the supporting electrolyte. The DP voltammogram obtained in these conditions, Fig. 5A, showed peaks 2a and 3a corresponding to the oxidation of the cur- cumin oxidation products. Also, the first DP voltammogram recorded in ferulic acid showed both oxidation peaks 1a and 2a, Fig. 5B, and after succes- sive DP voltammograms in the same solution, the oxidation prod- uct peak 3a, occurred. DP voltammograms in solutions of capsaicin or dihydrocapsai- cin showed only one charge transfer reaction correspondent to peak 1a, after successive DP voltammograms in the same solution, without cleaning the electrode surface, capsaicin peak 3a, Fig. 5C, appeared at a lower potential and the peak current increased with the number of scans. The pH study of peak 3a was also carried out on the second DP voltammograms in solutions of curcumin, Fig. 6. In each supporting On the first DP voltammograms recorded in all electrolytes cur- cumin and ferulic acid oxidation peaks 1a and 2a, occurred, and the peak currents were higher in acidic media, Figs. 3A and 4A. For 1.3 < pH < 9.0 both peak potentials were pH-dependent and following a linear relationship, Fig. 3B. The slope of the lines, 60 mV per pH unit indicates that the same number of proton and electron are involved in each redox reactions. The width at half- height of peak 1a, W1/2 80 mV, was close to the theoretical value for the transfer of one electron thus the oxidation of curcumin and ferulic acid involved in peak 1a the transfer of one electron and one proton. For peak 2a, W1/2 60 mV, the reaction involved the trans- fer of two electrons and two protons. For pH > 9.0, the oxidation processes was pH-independent, Fig. 3B, specific of an electrochemical reaction with the electron transfer after chemical deprotonation, enabling the determination of pKa 9.0. The oxidation of capsaicin and dihydrocapsaicin was also pH-dependent, Fig. 4B, on the whole pH interval, and the peak 1a potential variation linear. The slope of the line, 60 mV per pH unit, and the width at half-height of the peak 1a, W1/2 80 mV, indi- cated the transfer of one electron and one proton. DP voltammograms were successively recorded in all electro- lytes, Fig. 5. The first DP voltammogram recorded in a solution ofPDF Image | Guaicolic spices curcumin and capsaicin electrochemical oxidation
PDF Search Title:
Guaicolic spices curcumin and capsaicin electrochemical oxidationOriginal File Name Searched:
170-curcumin-JEAC.PDFDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |