
PDF Publication Title:
Text from PDF Page: 011
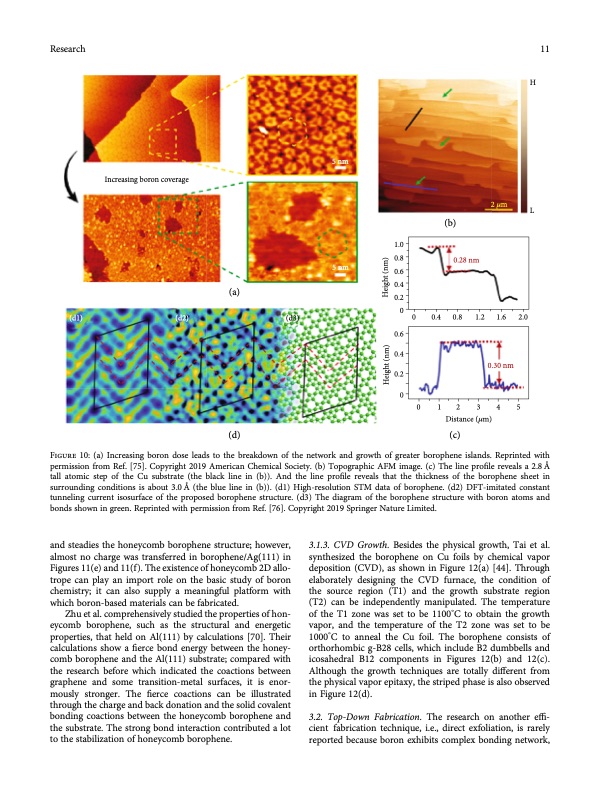
Research 11 Increasing boron coverage (a) (d2) (d3) (d) 5 nm 5 nm H 2𝜇m L (d1) 1.0 0.8 0.6 0.4 0.2 0 0.6 0.4 0.2 (b) 0 0.4 0.8 1.2 1.6 2.0 Figure 10: (a) Increasing boron dose leads to the breakdown of the network and growth of greater borophene islands. Reprinted with permission from Ref. [75]. Copyright 2019 American Chemical Society. (b) Topographic AFM image. (c) The line profile reveals a 2.8 Å tall atomic step of the Cu substrate (the black line in (b)). And the line profile reveals that the thickness of the borophene sheet in surrounding conditions is about 3.0 Å (the blue line in (b)). (d1) High-resolution STM data of borophene. (d2) DFT-imitated constant tunneling current isosurface of the proposed borophene structure. (d3) The diagram of the borophene structure with boron atoms and bonds shown in green. Reprinted with permission from Ref. [76]. Copyright 2019 Springer Nature Limited. and steadies the honeycomb borophene structure; however, almost no charge was transferred in borophene/Ag(111) in Figures 11(e) and 11(f). The existence of honeycomb 2D allo- trope can play an import role on the basic study of boron chemistry; it can also supply a meaningful platform with which boron-based materials can be fabricated. Zhu et al. comprehensively studied the properties of hon- eycomb borophene, such as the structural and energetic properties, that held on Al(111) by calculations [70]. Their calculations show a fierce bond energy between the honey- comb borophene and the Al(111) substrate; compared with the research before which indicated the coactions between graphene and some transition-metal surfaces, it is enor- mously stronger. The fierce coactions can be illustrated through the charge and back donation and the solid covalent bonding coactions between the honeycomb borophene and the substrate. The strong bond interaction contributed a lot to the stabilization of honeycomb borophene. 3.1.3. CVD Growth. Besides the physical growth, Tai et al. synthesized the borophene on Cu foils by chemical vapor deposition (CVD), as shown in Figure 12(a) [44]. Through elaborately designing the CVD furnace, the condition of the source region (T1) and the growth substrate region (T2) can be independently manipulated. The temperature of the T1 zone was set to be 1100°C to obtain the growth vapor, and the temperature of the T2 zone was set to be 1000°C to anneal the Cu foil. The borophene consists of orthorhombic g-B28 cells, which include B2 dumbbells and icosahedral B12 components in Figures 12(b) and 12(c). Although the growth techniques are totally different from the physical vapor epitaxy, the striped phase is also observed in Figure 12(d). 3.2. Top-Down Fabrication. The research on another effi- cient fabrication technique, i.e., direct exfoliation, is rarely reported because boron exhibits complex bonding network, 0.28 nm 0 012345 Distance (𝜇m) (c) 0.30 nm Height (nm) Height (nm)PDF Image | Two-Dimensional Borophene
PDF Search Title:
Two-Dimensional BoropheneOriginal File Name Searched:
borophene.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |