
PDF Publication Title:
Text from PDF Page: 032
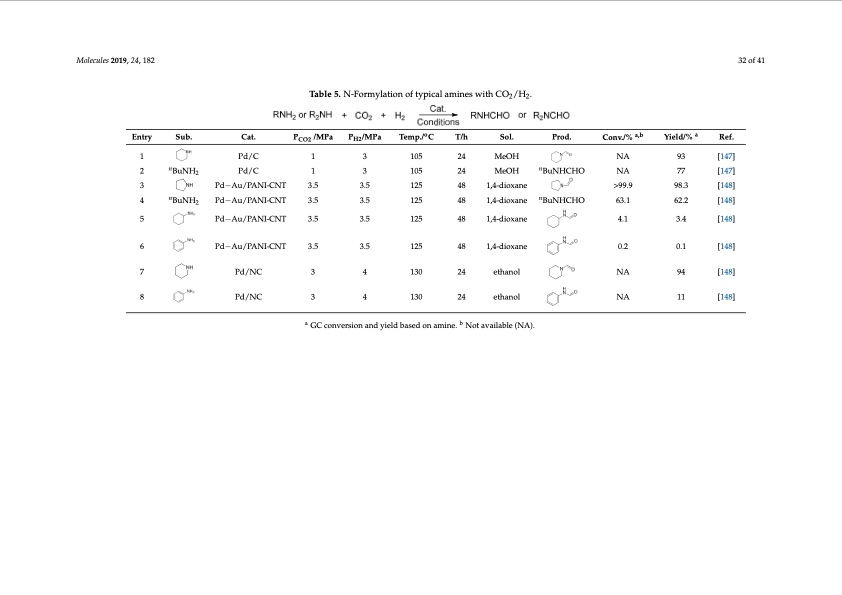
Molecules 201M9,o2le4c,uxleFsO20R1P9,E2E4R, xRFEOVRIEPWEER33REoVf I4E2W Molecules 201M9,o2le4c,uxleFsO20R1P9,E2E4R, xRFEOVRIEPWEER33REoVf I4E2W Molecules 201M9,o2le4c,uxleFsO20R19P,E2E4R, xRFEOVRIEPWEER33REoVf I4E2W Molecules 2019, 24, x FOR PEER REVIEW 33 of 42 Molecules 2019, 24, x FOR PEER REVIEW 33 of 42 33 of 42 33 of 42 33 of 42 Table 5. N-TFoabrmley5la. tNio-nForfmtyplaictiaolnamofintyepsiwcailthamCiOn2e/sHw2. ith CO2/H2. Table 5. N-Formylation of typical amines with CO2/H2. Table 5. N-Formylation of typical amines with CO2/H2. Table 5. N-TFoarbmley5la. tNio-nForfmtyplaictiaolnamofintyepsiwcailthamCiOn2e/sHw2. ith CO2/H2. Molecules 2019, 24, x FOR PEER REVIEW 33 of 42 Table 5. N-Formylation of typical amines with CO2/H2. Table 5. N-Formo ylation of otypical amines with CO2/H2. a,b a,b a PCrodn.v./% ConYvi./e%ld/% YieRledf/.% Entry SEunbt.ry Sub. Cat. Cat.PCO2 /MPa PCPOH2 2//MPa PTHe2m/MpP./aC TTem/hp./ C STo/lh. SolP. rod. a Ref. Ref. Molecules 2019, 24, 182 Entry Entry Entry 32 of 41 Sub. Cat. Sub. Cat. Pd/C Table 5. N-TFaobrmley5la. tNio-nFoorfmotyplaitciaolnaomfiotnyepsicwailthamCiOne2/sHw2.ith CO2/H2. PCO2 /MPa PH2/MPa Temp./ C T/h Sol. Prod. Conv./% a,b a,b a Yield/% Ref. Entry Sub. Cat. PCO2 /MPa PH2/MPa Temp./ C T/h Sol. Prod. Conv./% Yield/% a 1 P d / C P d / C 1 1 3 13 0 5 CatP. CO2 /TMaPbaleP5C.PONH2 2/-/MFMoPrPma ylaPTtHieo2m/nMpoPf./atCypiTcaTelm/ahmp.i/nCes wSTiot/lh. CO2/HS2. olP. rod. 1 2 14 0 5 M e 2O4 H M e O H oo NA NA93 a,b a,b a [19437] YieRledf/.% [147] SEunbt.ry 1 1 n Entry BSuuNbH. 2 Table 5.TNab-Fleo5rm. Ny-lFaotriomnyloaftitoynpoicfatylpaimcailnaemsiwneisthwCithOC2/OH22/.H2. PCrodn.v./% ConYvi.e/%ld/% a Ref. 1 1Table 5. N3-Formylat1io0n5 of typ2i4cal amMineOsHwith CO /H . Pd/C 1 3 105 24 MeO2H2 NA 93 [147] NA 93 [147] 2 o o P d / C 1 1 3 1 3 0 5 2 1 4 0 5 M e 2 O 4 H M B e Ou NH H C H O B u N H C N H A O N A 7 7 a Ref. n SEunbt.ry BuN2H2 n Sub. Cat. B u N H P 2 d / C a,b a,ba Cat.PCO2 /MPa PCPOH2 2//MPa PTHe2m/MpP./aC TTem/hp./ C STo/lh. nSolP. rod. n PCrodn.v./% ConYvi./e%ld/% YieRledf/.% [1747] 1 Pd/C Pd/C 1 21405 Me2O4H MeOH NA NA93 [19437] [147] Sub. Cat. mp./oC T/h nSol. Prod. 2/4h MSeoOl.H BuNPrHodC.HOn ConNvA./% a,bConv./% a,b Yie7ld7/% Yield/% a [147] En2try 1 2 n PCda/tC. BuNH2 PCO2 1/MPa Pd/C 105 24 MeOH BuNHCHO NA a [R14e7f.] 77 Ref. Pd/C [147] 3 1 Pd/C 1 21405 Me2O4H MeOH NA Pd−Au/PANPdI-−CANuT/PANI-C3N.5T 41825 1,4-di4o8xane1,4-dioxane >99.9 NA93 >99.98.3 [19437] [9184.83] [147] 2 3 BuNHP2d/C Pd/C 1 13 1305 o 21405 Me2O4H MBeuONHHCHOBuNHCNHAO a,b NA77 a [1747] [148] Entry BuN2H2 n Sub. n Cat. PCO2 /MPa PH2/MPa Temp./ C T/h o Sol. n Prod. n Conv./% Yielda/,b% Ref. a [147] Entry Teomp./ C T/h Sol. Prod. Conv./% Sub. Cat. P /MPa P /MPa Temp./o C T/h Sol. Prod. Conv./%a,b Sub.1 Pd−Au/PCPdAa/tCN. I-CNTPd/C PC3O1.252 /MPa 13P3.H52/MPa 1312T05emp./ 4C218405 T1/,h4M-dei2oO4xHaneSoMl. eOH Prod. >N99A.9 ConvN./A%9983.3 a,b Yield/% aRef. [14R7e] f. Entry Sub. Cat. PCO2 /MPa PH2/MPa Entry31 2 3 Pd−Au/PANI-CNT 3.5 3.5 125 48 1,4-dioxane >99.9 Yield/a% Yi[1e194l3d87]/% Ref. 1 13 1305 21405 nn 98.3 [148] nBuN2H2 BuNHP2 d/C Pd/C Me2O4H MnBeOuNHHCHOBuNHCNHAO NA77 [1747] 4 nn nn [147] BuN4H2 PBdu−NAHu/2PANPdI-−CANuT/PANI-C3N.5T 3.35.5 31.525 41825 1,4-di4o8xane1,4-dBiuoxNaHneCHOBuNHC6H3.O1 63.162.2 [6124.82] [148] 3 3 P d − A u / P A N P d I - − C A N u T / P A N I - C 3 N. 5 T 3 . 3 5 . 5 31.525 41825 1,4-di4o8xane1,4-dioxane >99.9 >99.98.3 [9184.83] [147] 93 [148] 1 1 PPdd/C/C 1 1 3 3 105 105 24 M24eOH MeOH NA N9A3 [147] [ 1 [4 18 4] 7 ] 1 4 BuNHP2 d/CPd−Au/PANI-CNT1 3 125 2 nn nn nBuN2H2 BuNH2 nBuNHP2 d/C Pd/C 1 Pd−Au/PANI-CNT 3.5 13 3.5 3105 2 1 4 0 5 M e 2 O 4 H M n B e Ou NH H C H On B u N H C N H A O NA 77 48 1,4-dioxane BuNHCHO 63.1 62.2 [17477] [148] 629.23 [147] 1 Pd/C 1 3 105 24 MeOH NA 125 24 48 M1e,O4-Hdioxane nBuNHCHO N63A.1 93 [147] 3 n4 Pd−Au/PANPdI-−CANuT/PANI-C3N.5T Pd/C 1 3.5 3.5 105 41825 1,4-di4o8xane1,4-dioxane >99.9 >99.98.3 24 MeOH BuNHCHO NA 2 3 3 3.35.5 3.35.5 31.525 31.525 [9184.83] [134.48] 77 [148] 5 BuNH2 Pn d−Au/PANPdI-−CANuT/PANI-C3N.5T n P B d u − NA Hu / P 2 P d A / C NP d I - − C A N u T / P A N I - C 3 1 N. 5 T 3 3.35.5 3.5 105 31.525 125 41825 1,4-di4o8xane1,4-ndioxane n 4.1 4.1 3.4 4218425 1,4M-dei4Oo8xHane1,4-dBiuoxNaHneCHOn BuNHC6NH3A.1O 63.16727.2 [147] [148] 42 n 5 BuN4H2 3.353.5 1 31.5205 3 [6124.872] 77 [148] 2 BuNH2 Pd/C 105 24 MeOH BuNHCHO NA [147] 3 3 PdP−dA−uA/uP/APNPAdIN-−CAI-NCuT/NPTANI-C3N.35.T5 Pn d−AuP/PdA/CNPdI-−CANuT/PANI-C3N.5T1 41825 1,44-d8i4o8xane1,4-dioxane >99.9 >9>9.98..93 41825 21,4-di4o8xaMne1e,O4-HndioxanenBunNHCH4O.1 N 4 A. 1 3 . 4 [9184.83]98.3 [1347.487] [148] [148] [ 1 [4 18 4] 7 ] 2 5 3.35.5 3 3.5 31.525 105 4 n BuNH PBdu−NAHu/2PANPdI-−CANuT/PANI-C3N.5T 3.35.5 3.5 31.525 125 41825 1,4-di4o8xane1,4-dBiuoxNaHneCHOBuNHC6H3.O1 63.162.2 48 1,4-dioxane n BuNHCHO 63.1 [6124.82] [148] nBnuNH52 BuN4H2 6 PPd−Au/PANPNdII-−-CANuT/TPANI-C3N.53T.5 3.35.5 3.5 31.525 5 PdP−dA−uA/PuA/PNPAdIN-−CAIN-CuT/NPTANI-C3N.35.T5 3.35.5 3.5 31.525 4 3 Pd−Au/PANI-CNT 3.5 Pd−Au/PANI-CNT 3.5 Pd−Au/PANPdI-−CANuT/PANI-C3N.5T 125 48 1,4-dioxane >99.9 98.3 62.2 [148] [148] 5 4.1 >949.1.93.4 [91384..483] [6124.82] [148] [ 1 [4 18 4] 8 ] 2 [148] 35 BuN4H2 PBdu−NAHu/2PANPdI-−CANuT/PANI-C3N.5T 3.35.5 31.525 3.35.5 31.525 nn nn 41825 1,4-di4o8xane1,4-dioxane 41825 1,4-di4o8xane1,4-dBiouxNaHneCHOBuNHC6H3.O1 63.162.2 3 4 125 41825 41,84-di4o81x,a4n-ed1i,o4-xdainoxeane 0.2 >909..290.1 41825 1,4-4d8i4o8xane1,,4-diioxane 4.1 4.143.1.4 [1094.818.]3 [134.48]3.4 5 6 125 [148] [148] [148] 5 4 nBuNH2 nPd−Au/PANI-CNT 3.5 3.5 125 48 1,4-dioxane nBuNHCHOn 63.1 62.2 125 48 1,4-dioxane BuNHCHO 63.1 [148] 6 4 BuNH2 Pd−Au/PANI-CNT 3.5 3.5 62.2 Pd−Au/PANI-CNT 3.5 3.5 125 48 1,4-dioxane 0.2 0.1 [148] 6 Pd−Au/PANI-CNT 3.5 3.5 125 48 1,4-dioxane 0.2 0.1 [148] 4 5 5 Pd−Au/PANPdI-−CANuT/PANI-C3N.5T 3.35.5 31.525 nBuNH2 Pd−Au/PANI-CNT 3.5 3.5 125 41825 1,4-di4o8xane1,4-dioxane 4.1 4.1 3.4 48 1,4-dioxane nBuNHCHO 63.1 [134.48] 62.2 [148] 6 6 5 7 6 57 8 6 7 8 6 PdP−dA−uA/PuA/PNPAdIN-−CAIN-CuT/NPTANI-C3N.35.T5 3.35.5 3.5 31.525 41825 1,4-4d8i4o8xane1,,4-diioxane 0.2 0.200.2.1 [104.18] 0.1 [148] [148] Pd−Au/PANI-CNT 3.5 3.5 125 125 48 1,4-dioxane 4.1 3.4 [148] [148] [148] 5 Pd−Au/PANI-CNT 3.5 3.5 125 48 1,4-dioxane 4.1 3.4 7 P d / N C P d / N C 3 3 4 14 3 0 6 Pd−Au/PANPdI-−CANuT/PANI-C3N.5T 3.35.5 31.525 21430 etha2n4ol ethanol NA NA94 41825 1,4-di4o8xane1,4-dioxane 0.2 0.2 0.1 [19448] [104.18] [148] [148] 7 Pd−AuP/PdPd/AN/NCCI-CNT 33.5 7 Pd/NC 3 4 34.5 130 4 112350 130 24 48e2th4an1o,4l -dioetxhaaneol NA 4.1N9A4 [1348.4] 94 94 [148[]148] [148] 6 Pd−Au/PANPdI-−CANuT/PANI-C3N.5T 3.35.5 31.525 7 7 Pd/NC Pd/NC 3 34 1430 130 24 ethanol NA 6 [148] 41825 1,4-di4o8xane1,4-dioxane 0.2 0.2 0.1 [104.18] NA NA94 [19448] [148] 6 6 Pd−Au/PANI-CNT 3.5 3.5 125 21430 etha2n4ol ethanol 48 1,4-dioxane 0.2 0.1 [148] 8 8 PPdd/N/NCC Pd/NC 3 3 34 4 1430 P d / N C P d / N C 3 3 4 14 3 0 21430 e2th4a2n4ol ethanol NA 21430 etha2n4ol ethanol NA NAN1A1 NA94 [11418] 11 [19448] [148] [148] [148] Pd−Au/PANI-CNT 3.5 3.5 125 48 1,4-dioxane 0.2 0.1 [148] 13 1305 PCPO2H2//MPaa PTHe2/mMpP./aC TeT 3 105 o 13 1 3 13 0 5 3.35.5 31.525 7 7 8 Pd/NC 3 4 130 24 ethanol NA 11 [148] 8 Pd/NC 3 4 130 24 ethanol NA 11 [148] 7 P d / N C P d / N C 3 3 4 21430 eth2a4nol ethanol NA 48 1,4-dioxane 7 41 3 0 aaa bbb NA 94 0.2 [1948] 0.1 [148] Pd−Au/PANI-CNT 3.5 3.5 125 [148] 8 7 8 8 8 8 Pd/NC Pd/NC 3GGCCcocnovnevrseir3Gos4niCoancnodannyvdieerlysdioeb14nlad3sa0ebndasoyenidealodm2nbi14na3esm0.edinNoeone.ttahamvNa2ain4noioletal.balveNa(oieNltatAhabval)ae.niol(aNlbAle)(.NA). NA NA11 94 [11418] [148] [148] Pd/NC Pd/NC Pd/NC Pd/NC Pd/NC Pd/NC a Pd/NC Pd/NC 3 4 130 24 ethanol NA 7 3 4 130b 24 ethanol NA 94 [148] 8 3GC conversi3o4n and yield b14a3s0ed on am2i14n3e0. Noetthava2an4iolalble (eNtAha).nol NA a GC conversion and yield based on amine. b Not available (NA). NA11 [11418] [148] aa bb GC conversiGonCacnodnyvierlsdiobnasaendoyniealmd binaes.edNoontamvainilea.bleN(oNt Aav)a. ilable (NA). P d / N C P d / N C 3 3 4 41 3 0 2 14 3 0 e t h 2a 4n o l e t h a n o l 8 8 3 4 130 24 ethanol NA NA 11 NA 11 [1418] 94 [148] aa bb GC conversiGonCacnodnyvieerlsdiobnasaendoyniealdmbinaes.edNoontaamvainilea.bleN(oNt Aav)a.ilable (NA). 3 4 130 24 ethanol NA [148] 3 4 130 24 ethanol aa bb GC conversiGonCacnodnvyeierlsdiobnaasend oyniealdmbinaes.edNonotaamvianilea.blNe (oNt aAv)a.ilable (NA). NA NA [148] [148] [148] 11 a GC conversaion and yield based on amine. b Not availabble (NA). GC conversion and yield based on amine. Not available (NA). 3 4 130 24 ethanol 11 a GC conversion and yield based on amine. b Not available (NA).PDF Image | Catalytic Conversion of Carbon Dioxide through C-N Bond
PDF Search Title:
Catalytic Conversion of Carbon Dioxide through C-N BondOriginal File Name Searched:
molecules-24-00182.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |