
PDF Publication Title:
Text from PDF Page: 033
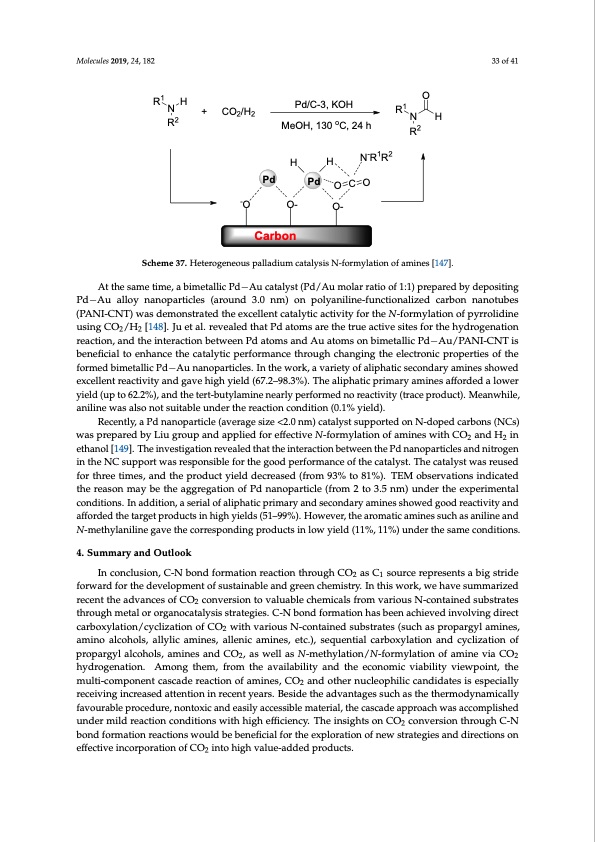
Molecules 2019, 24, 182 Molecules 2019, 24, x FOR PEER REVIEW 33 of 41 R1 H N + CO2/H2 R2 -O O- O- Carbon O 34 of 42 Pd HH Pd O C O Pd/C-3, KOH MeOH, 130 oC, 24 h R1 N R2 H N-R1R2 Scheme 37. Heterogeneous palladium catalysis N-formylation of amines [147]. Scheme 37. Heterogeneous palladium catalysis N-formylation of amines [147]. At the same time, a bimetallic Pd−Au catalyst (Pd/Au molar ratio of 1:1) prepared by depositing At the same time, a bimetallic Pd−Au catalyst (Pd/Au molar ratio of 1:1) prepared by depositing Pd−Au alloy nanoparticles (around 3.0 nm) on polyaniline-functionalized carbon nanotubes Pd−Au alloy nanoparticles (around 3.0 nm) on polyaniline-functionalized carbon nanotubes (PANI-CNT) was demonstrated the excellent catalytic activity for the N-formylation of pyrrolidine (PANI-CNT) was demonstrated the excellent catalytic activity for the N-formylation of pyrrolidine usingCO/H [148].Juetal.revealedthatPdatomsarethetrueactivesitesforthehydrogenation 22 using CO2/H2 [148]. Ju et al. revealed that Pd atoms are the true active sites for the hydrogenation reaction, and the interaction between Pd atoms and Au atoms on bimetallic Pd−Au/PANI-CNT is reaction, and the interaction between Pd atoms and Au atoms on bimetallic Pd−Au/PANI-CNT is beneficial to enhance the catalytic performance through changing the electronic properties of the beneficial to enhance the catalytic performance through changing the electronic properties of the formed bimetallic Pd−Au nanoparticles. In the work, a variety of aliphatic secondary amines showed formed bimetallic Pd−Au nanoparticles. In the work, a variety of aliphatic secondary amines showed excellent reactivity and gave high yield (67.2–98.3%). The aliphatic primary amines afforded a lower excellent reactivity and gave high yield (67.2–98.3%). The aliphatic primary amines afforded a lower yield (up to 62.2%), and the tert-butylamine nearly performed no reactivity (trace product). Meanwhile, yield (up to 62.2%), and the tert-butylamine nearly performed no reactivity (trace product). aniline was also not suitable under the reaction condition (0.1% yield). Meanwhile, aniline was also not suitable under the reaction condition (0.1% yield). Recently, a Pd nanoparticle (average size <2.0 nm) catalyst supported on N-doped carbons (NCs) Recently, a Pd nanoparticle (average size <2.0 nm) catalyst supported on N-doped carbons was prepared by Liu group and applied for effective N-formylation of amines with CO2 and H2 in (NCs) was prepared by Liu group and applied for effective N-formylation of amines with CO2 and ethanol [149]. The investigation revealed that the interaction between the Pd nanoparticles and nitrogen H2 in ethanol [149]. The investigation revealed that the interaction between the Pd nanoparticles and in the NC support was responsible for the good performance of the catalyst. The catalyst was reused nitrogen in the NC support was responsible for the good performance of the catalyst. The catalyst for three times, and the product yield decreased (from 93% to 81%). TEM observations indicated was reused for three times, and the product yield decreased (from 93% to 81%). TEM observations the reason may be the aggregation of Pd nanoparticle (from 2 to 3.5 nm) under the experimental indicated the reason may be the aggregation of Pd nanoparticle (from 2 to 3.5 nm) under the conditions. In addition, a serial of aliphatic primary and secondary amines showed good reactivity and experimental conditions. In addition, a serial of aliphatic primary and secondary amines showed afforded the target products in high yields (51–99%). However, the aromatic amines such as aniline and good reactivity and afforded the target products in high yields (51–99%). However, the aromatic N-methylaniline gave the corresponding products in low yield (11%, 11%) under the same conditions. amines such as aniline and N-methylaniline gave the corresponding products in low yield (11%, 11%) under the same conditions. 4. Summary and Outlook 4. SuImnmcoanrycluansidonO,uCtl-oNokbond formation reaction through CO2 as C1 source represents a big stride forward for the development of sustainable and green chemistry. In this work, we have summarized In conclusion, C-N bond formation reaction through CO2 as C1 source represents a big stride recent the advances of CO2 conversion to valuable chemicals from various N-contained substrates forward for the development of sustainable and green chemistry. In this work, we have summarized through metal or organocatalysis strategies. C-N bond formation has been achieved involving direct recent the advances of CO2 conversion to valuable chemicals from various N-contained substrates carboxylation/cyclization of CO2 with various N-contained substrates (such as propargyl amines, through metal or organocatalysis strategies. C-N bond formation has been achieved involving direct amino alcohols, allylic amines, allenic amines, etc.), sequential carboxylation and cyclization of carboxylation/cyclization of CO2 with various N-contained substrates (such as propargyl amines, propargyl alcohols, amines and CO2, as well as N-methylation/N-formylation of amine via CO2 amino alcohols, allylic amines, allenic amines, etc.), sequential carboxylation and cyclization of hydrogenation. Among them, from the availability and the economic viability viewpoint, the propargyl alcohols, amines and CO2, as well as N-methylation/N-formylation of amine via CO2 multi-component cascade reaction of amines, CO2 and other nucleophilic candidates is especially hydrogenation. Among them, from the availability and the economic viability viewpoint, the receiving increased attention in recent years. Beside the advantages such as the thermodynamically multi-component cascade reaction of amines, CO2 and other nucleophilic candidates is especially favourable procedure, nontoxic and easily accessible material, the cascade approach was accomplished receiving increased attention in recent years. Beside the advantages such as the thermodynamically under mild reaction conditions with high efficiency. The insights on CO2 conversion through C-N favourable procedure, nontoxic and easily accessible material, the cascade approach was bond formation reactions would be beneficial for the exploration of new strategies and directions on accomplished under mild reaction conditions with high efficiency. The insights on CO2 conversion effective incorporation of CO2 into high value-added products. Gratifyingly, encouraging advances on the development of novel strategies to construct organic molecules through C-N bond formation have been made, however, many problems and challenges through C-N bond formation reactions would be beneficial for the exploration of new strategies and directions on effective incorporation of CO2 into high value-added products.PDF Image | Catalytic Conversion of Carbon Dioxide through C-N Bond
PDF Search Title:
Catalytic Conversion of Carbon Dioxide through C-N BondOriginal File Name Searched:
molecules-24-00182.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |