
PDF Publication Title:
Text from PDF Page: 002
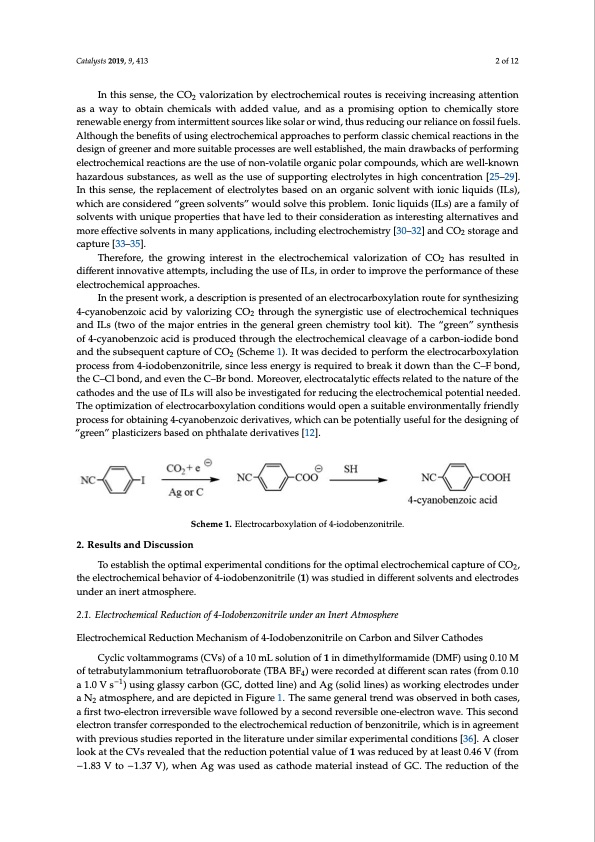
Catalysts 2019, 9, 413 2 of 12 In this sense, the CO2 valorization by electrochemical routes is receiving increasing attention as a way to obtain chemicals with added value, and as a promising option to chemically store renewable energy from intermittent sources like solar or wind, thus reducing our reliance on fossil fuels. Although the benefits of using electrochemical approaches to perform classic chemical reactions in the design of greener and more suitable processes are well established, the main drawbacks of performing electrochemical reactions are the use of non-volatile organic polar compounds, which are well-known hazardous substances, as well as the use of supporting electrolytes in high concentration [25–29]. In this sense, the replacement of electrolytes based on an organic solvent with ionic liquids (ILs), which are considered “green solvents” would solve this problem. Ionic liquids (ILs) are a family of solvents with unique properties that have led to their consideration as interesting alternatives and more effective solvents in many applications, including electrochemistry [30–32] and CO2 storage and capture [33–35]. Therefore, the growing interest in the electrochemical valorization of CO2 has resulted in different innovative attempts, including the use of ILs, in order to improve the performance of these electrochemical approaches. In the present work, a description is presented of an electrocarboxylation route for synthesizing 4-cyanobenzoic acid by valorizing CO2 through the synergistic use of electrochemical techniques and ILs (two of the major entries in the general green chemistry tool kit). The “green” synthesis of 4-cyanobenzoic acid is produced through the electrochemical cleavage of a carbon-iodide bond and the subsequent capture of CO2 (Scheme 1). It was decided to perform the electrocarboxylation process from 4-iodobenzonitrile, since less energy is required to break it down than the C–F bond, the C–Cl bond, and even the C–Br bond. Moreover, electrocatalytic effects related to the nature of the cathodes and the use of ILs will also be investigated for reducing the electrochemical potential needed. The optimization of electrocarboxylation conditions would open a suitable environmentally friendly process for obtaining 4-cyanobenzoic derivatives, which can be potentially useful for the designing of “green” plasticizers based on phthalate derivatives [12]. Scheme 1. Electrocarboxylation of 4-iodobenzonitrile. 2. Results and Discussion To establish the optimal experimental conditions for the optimal electrochemical capture of CO2, the electrochemical behavior of 4-iodobenzonitrile (1) was studied in different solvents and electrodes under an inert atmosphere. 2.1. Electrochemical Reduction of 4-Iodobenzonitrile under an Inert Atmosphere Electrochemical Reduction Mechanism of 4-Iodobenzonitrile on Carbon and Silver Cathodes Cyclic voltammograms (CVs) of a 10 mL solution of 1 in dimethylformamide (DMF) using 0.10 M of tetrabutylammonium tetrafluoroborate (TBA BF4) were recorded at different scan rates (from 0.10 a 1.0 V s−1) using glassy carbon (GC, dotted line) and Ag (solid lines) as working electrodes under a N2 atmosphere, and are depicted in Figure 1. The same general trend was observed in both cases, a first two-electron irreversible wave followed by a second reversible one-electron wave. This second electron transfer corresponded to the electrochemical reduction of benzonitrile, which is in agreement with previous studies reported in the literature under similar experimental conditions [36]. A closer look at the CVs revealed that the reduction potential value of 1 was reduced by at least 0.46 V (from −1.83 V to −1.37 V), when Ag was used as cathode material instead of GC. The reduction of thePDF Image | Electrocatalytic Processes for the Valorization of CO2
PDF Search Title:
Electrocatalytic Processes for the Valorization of CO2Original File Name Searched:
catalysts-09-00413.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |