
PDF Publication Title:
Text from PDF Page: 003
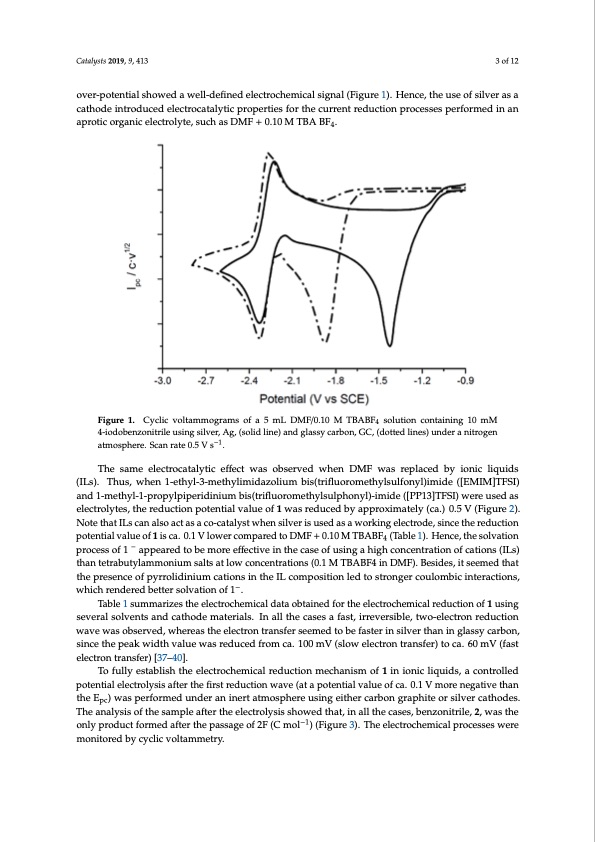
electron transfer corresponded to the electrochemical reduction of benzonitrile, which is in agreement with previous studies reported in the literature under similar experimental conditions [36]. A closer look at the CVs revealed that the reduction potential value of 1 was reduced by at least 0.46 V (from −1.83 V to −1.37 V), when Ag was used as cathode material instead of GC. The reduction of the over- Catalysts 2019, 9, 413 3 of 12 potential showed a well-defined electrochemical signal (Figure 1). Hence, the use of silver as a cathode introduced electrocatalytic properties for the current reduction processes performed in an aprotic organic electrolyte, such as DMF + 0.10 M TBA BF4. over-potential showed a well-defined electrochemical signal (Figure 1). Hence, the use of silver as a cathode introduced electrocatalytic properties for the current reduction processes performed in an aprotic organic electrolyte, such as DMF + 0.10 M TBA BF4. Figure 1. Cyclic voltammograms of a 5 mL DMF/0.10 M TBABF4 solution containing 10 mM 4-iodobenzonitrile using silver, Ag, (solid line) and glassy carbon, GC, (dotted lines) under a nitrogen Figure 1. Cyclic voltammograms of a 5 mL DMF/0.10 M TBABF4 solution containing 10 mM 4- atmosphere. Scan rate 0.5 V s−1. iodobenzonitrile using silver, Ag, (solid line) and glassy carbon, GC, (dotted lines) under a nitrogen atmosphere. Scan rate 0.5 V s−1. The same electrocatalytic effect was observed when DMF was replaced by ionic liquids (ILs). Thus, when 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM]TFSI) The same electrocatalytic effect was observed when DMF was replaced by ionic liquids (ILs). and 1-methyl-1-propylpiperidinium bis(trifluoromethylsulphonyl)-imide ([PP13]TFSI) were used as Thus, when 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM]TFSI) and 1- electrolytes, the reduction potential value of 1 was reduced by approximately (ca.) 0.5 V (Figure 2). methyl-1-propylpiperidinium bis(trifluoromethylsulphonyl)-imide ([PP13]TFSI) were used as Note that ILs can also act as a co-catalyst when silver is used as a working electrode, since the reduction electrolytes, the reduction potential value of 1 was reduced by approximately (ca.) 0.5 V (Figure 2). potential value of 1 is ca. 0.1 V lower compared to DMF + 0.10 M TBABF4 (Table 1). Hence, the solvation Note that ILs can also act as a co-catalyst when silver is used as a working electrode, since the process of 1 − appeared to be more effective in the case of using a high concentration of cations (ILs) reduction potential value of 1 is ca. 0.1 V lower compared to DMF + 0.10 M TBABF4 (Table 1). Hence, than tetrabutylammonium salts at low concentrations (0.1 M TBABF4 in DMF). Besides, it seemed that the solvation process of 1 − appeared to be more effective in the case of using a high concentration of the presence of pyrrolidinium cations in the IL composition led to stronger coulombic interactions, cations (ILs) than tetrabutylammonium salts at low concentrations (0.1 M TBABF4 in DMF). Besides, which rendered better solvation of 1−. it seemed that the presence of pyrrolidinium cations in the IL composition led to stronger coulombic Table 1 summarizes the electrochemical data obtained for the electrochemical reduction of 1 using interactions, which rendered better solvation of 1−. several solvents and cathode materials. In all the cases a fast, irreversible, two-electron reduction wave was observed, whereas the electron transfer seemed to be faster in silver than in glassy carbon, since the peak width value was reduced from ca. 100 mV (slow electron transfer) to ca. 60 mV (fast electron transfer) [37–40]. To fully establish the electrochemical reduction mechanism of 1 in ionic liquids, a controlled potential electrolysis after the first reduction wave (at a potential value of ca. 0.1 V more negative than the Epc) was performed under an inert atmosphere using either carbon graphite or silver cathodes. The analysis of the sample after the electrolysis showed that, in all the cases, benzonitrile, 2, was the only product formed after the passage of 2F (C mol−1) (Figure 3). The electrochemical processes were monitored by cyclic voltammetry.PDF Image | Electrocatalytic Processes for the Valorization of CO2
PDF Search Title:
Electrocatalytic Processes for the Valorization of CO2Original File Name Searched:
catalysts-09-00413.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |