
PDF Publication Title:
Text from PDF Page: 002
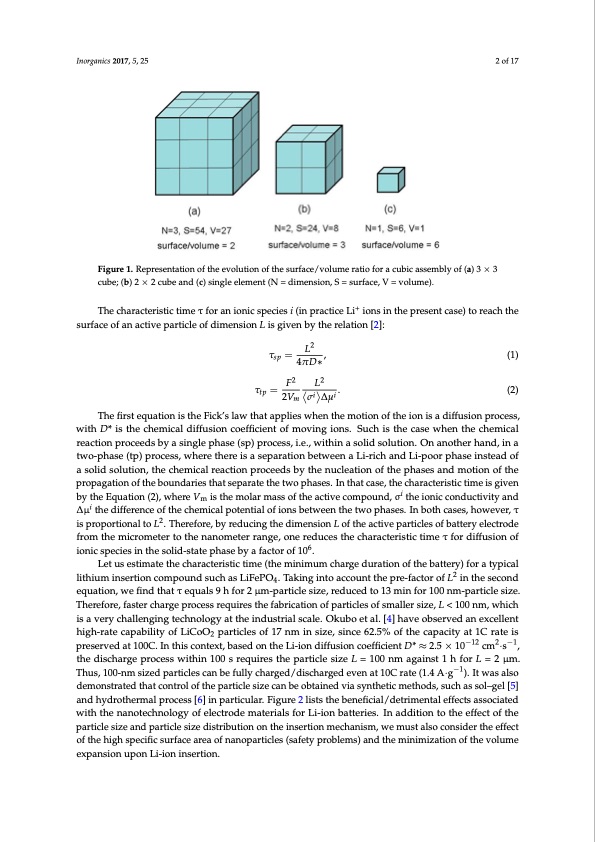
Inorganics 2017, 5, 25 2 of 17 Inorganics 2017, 5, 25 2 of 17 Figure 1. Representation of the evolution of the surface/volume ratio for a cubic assembly of (a) 3 × 3 Figure 1. Representation of the evolution of the surface/volume ratio for a cubic assembly of (a) 3 × 3 cube; (b) 2 × 2 cube and (c) single element (N = dimension, S = surface, V = volume). cube; (b) 2 × 2 cube and (c) single element (N = dimension, S = surface, V = volume). The characteristic time τ for an ionic species i (in practice Li+ ions in the present case) to reach The characteristic time τ for an ionic species i (in practice Li+ ions in the present case) to reach the the surface of an active particle of dimension L is given by the relation [2]: surface of an active particle of dimension L is given by the relation [2]: τ = L2 , sp L2 (1) τsp = 4πD*, (1) 4πD∗ F2 L2 τtp= F2 L2 . (2) τtp=2V i i. m σi Δμi (2) The first equation is the Fick’s law that applies when the motion of the ion is a diffusion process, 2Vm σ ∆μ The first equation is the Fick’s law that applies when the motion of the ion is a diffusion process, with D* is the chemical diffusion coefficient of moving ions. Such is the case when the chemical with D* is the chemical diffusion coefficient of moving ions. Such is the case when the chemical reaction proceeds by a single phase (sp) process, i.e., within a solid solution. On another hand, in a reaction proceeds by a single phase (sp) process, i.e., within a solid solution. On another hand, in a two-phase (tp) process, where there is a separation between a Li-rich and Li-poor phase instead of two-phase (tp) process, where there is a separation between a Li-rich and Li-poor phase instead of a a solid solution, the chemical reaction proceeds by the nucleation of the phases and motion of the solid solution, the chemical reaction proceeds by the nucleation of the phases and motion of the propagation of the boundaries that separate the two phases. In that case, the characteristic time is given propagation of the boundaries that separate the two phases. In that case, the characteristic time is by the Equation (2), where Vm is the molar mass of the active compound, σi the ionic conductivity and given by the Equation (2), where Vm is the molar mass of the active compound, σi the ionic ∆μi the difference of the chemical potential of ions between the two phases. In both cases, however, τ conductivity and Δμi the difference of the chemical potential of ions between the two phases. In both is proportional to L2. Therefore, by reducing the dimension L of the active particles of battery electrode cases, however, τ is proportional to L2. Therefore, by reducing the dimension L of the active particles from the micrometer to the nanometer range, one reduces the characteristic time τ for diffusion of of battery electrode from the micrometer to the nanometer range, one reduces the characteristic time ionic species in the solid-state phase by a factor of 106. τ for diffusion of ionic species in the solid-state phase by a factor of 106. Let us estimate the characteristic time (the minimum charge duration of the battery) for a typical Let us estimate the characteristic time (the minimum charge duration of the battery) for a typical lithium insertion compound such as LiFePO . Taking into account the pre-factor of L2 in the second 42 lithium insertion compound such as LiFePO4. Taking into account the pre-factor of L in the second equation, we find that τ equals 9 h for 2 μm-particle size, reduced to 13 min for 100 nm-particle size. equation, we find that τ equals 9 h for 2 μm-particle size, reduced to 13 min for 100 nm-particle size. Therefore, faster charge process requires the fabrication of particles of smaller size, L < 100 nm, which Therefore, faster charge process requires the fabrication of particles of smaller size, L < 100 nm, which is a very challenging technology at the industrial scale. Okubo et al. [4] have observed an excellent is a very challenging technology at the industrial scale. Okubo et al. [4] have observed an excellent high-rate capability of LiCoO2 particles of 17 nm in size, since 62.5% of the capacity at 1C rate is high-rate capability of LiCoO2 particles of 17 nm in size, since 62.5% of the capacity at 1C rate is preserved at 100C. In this context, based on the Li-ion diffusion coefficient D* ≈ 2.5 × 10−12 cm2·s−1, preserved at 100C. In this context, based on the Li-ion diffusion coefficient D* ≈ 2.5 × 10−12 cm2·s−1, the the discharge process within 100 s requires the particle size L = 100 nm against 1 h for L = 2 μm. discharge process within 100 s requires the particle size L = 100 nm against 1 h for L = 2 μm. Thus, Thus, 100-nm sized particles can be fully charged/discharged even at 10C rate (1.4 A·g−1). It was also 100-nm sized particles can be fully charged/discharged even at 10C rate (1.4 A·g−1). It was also demonstrated that control of the particle size can be obtained via synthetic methods, such as sol–gel [5] demonstrated that control of the particle size can be obtained via synthetic methods, such as sol–gel and hydrothermal process [6] in particular. Figure 2 lists the beneficial/detrimental effects associated [5] and hydrothermal process [6] in particular. Figure 2 lists the beneficial/detrimental effects with the nanotechnology of electrode materials for Li-ion batteries. In addition to the effect of the associated with the nanotechnology of electrode materials for Li-ion batteries. In addition to the effect particle size and particle size distribution on the insertion mechanism, we must also consider the effect of the particle size and particle size distribution on the insertion mechanism, we must also consider of the high specific surface area of nanoparticles (safety problems) and the minimization of the volume the effect of the high specific surface area of nanoparticles (safety problems) and the minimization of expansion upon Li-ion insertion. the volume expansion upon Li-ion insertion.PDF Image | Nanotechnology of Positive Electrodes for Li-Ion Batteries
PDF Search Title:
Nanotechnology of Positive Electrodes for Li-Ion BatteriesOriginal File Name Searched:
inorganics-05-00025.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |