
PDF Publication Title:
Text from PDF Page: 007
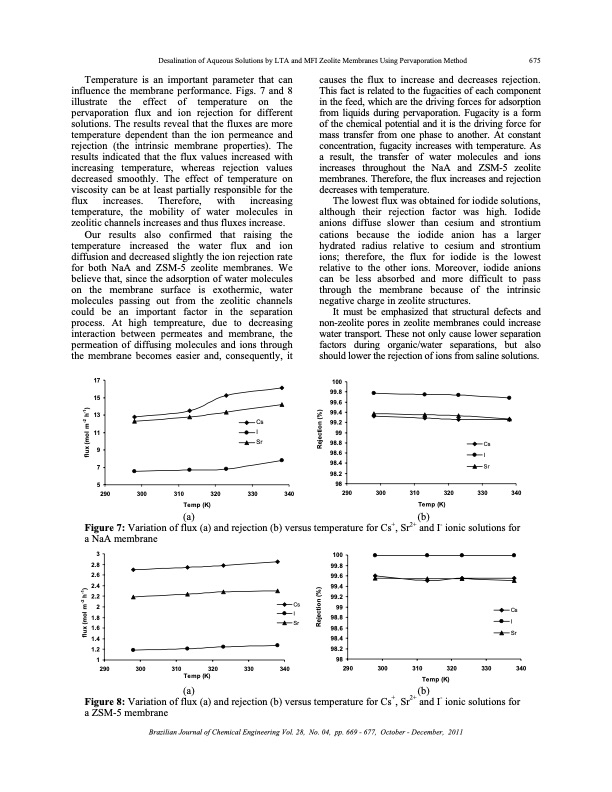
Desalination of Aqueous Solutions by LTA and MFI Zeolite Membranes Using Pervaporation Method 675 Temperature is an important parameter that can influence the membrane performance. Figs. 7 and 8 illustrate the effect of temperature on the pervaporation flux and ion rejection for different solutions. The results reveal that the fluxes are more temperature dependent than the ion permeance and rejection (the intrinsic membrane properties). The results indicated that the flux values increased with increasing temperature, whereas rejection values decreased smoothly. The effect of temperature on viscosity can be at least partially responsible for the flux increases. Therefore, with increasing temperature, the mobility of water molecules in zeolitic channels increases and thus fluxes increase. Our results also confirmed that raising the temperature increased the water flux and ion diffusion and decreased slightly the ion rejection rate for both NaA and ZSM-5 zeolite membranes. We believe that, since the adsorption of water molecules on the membrane surface is exothermic, water molecules passing out from the zeolitic channels could be an important factor in the separation process. At high tempreature, due to decreasing interaction between permeates and membrane, the permeation of diffusing molecules and ions through the membrane becomes easier and, consequently, it causes the flux to increase and decreases rejection. This fact is related to the fugacities of each component in the feed, which are the driving forces for adsorption from liquids during pervaporation. Fugacity is a form of the chemical potential and it is the driving force for mass transfer from one phase to another. At constant concentration, fugacity increases with temperature. As a result, the transfer of water molecules and ions increases throughout the NaA and ZSM-5 zeolite membranes. Therefore, the flux increases and rejection decreases with temperature. The lowest flux was obtained for iodide solutions, although their rejection factor was high. Iodide anions diffuse slower than cesium and strontium cations because the iodide anion has a larger hydrated radius relative to cesium and strontium ions; therefore, the flux for iodide is the lowest relative to the other ions. Moreover, iodide anions can be less absorbed and more difficult to pass through the membrane because of the intrinsic negative charge in zeolite structures. It must be emphasized that structural defects and non-zeolite pores in zeolite membranes could increase water transport. These not only cause lower separation factors during organic/water separations, but also should lower the rejection of ions from saline solutions. 100 99.8 99.6 99.4 99.2 99 98.8 98.6 98.4 98.2 98 290 300 310 320 Temp (K) Cs I Sr 330 340 17 15 13 11 9 7 5 290 300 310 320 Temp (K) Cs I Sr 330 340 (a) Figure 7: Variation of flux (a) and rejection (b) versus temperature for Cs+, Sr2+ and I- ionic solutions for a NaA membrane (b) 100 99.8 99.6 99.4 99.2 99 98.8 98.6 98.4 98.2 98 290 300 310 320 Temp (K) Cs I Sr 330 340 3 2.8 2.6 2.4 2.2 2 1.8 1.6 1.4 1.2 1 290 300 Cs I Sr 310 320 Temp (K) 330 340 (a) (b) Figure 8: Variation of flux (a) and rejection (b) versus temperature for Cs+, Sr2+ and I- ionic solutions for a ZSM-5 membrane Brazilian Journal of Chemical Engineering Vol. 28, No. 04, pp. 669 - 677, October - December, 2011 flux (mol m-2 h-1) flux (mol m-2 h-1) Rejection (%) Rejection (%)PDF Image | DESALINATION OF AQUEOUS SOLUTIONS ZEOLITE MEMBRANES
PDF Search Title:
DESALINATION OF AQUEOUS SOLUTIONS ZEOLITE MEMBRANESOriginal File Name Searched:
PCZPTXXvZ6pQgGbDbcz9VXd.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |