
PDF Publication Title:
Text from PDF Page: 002
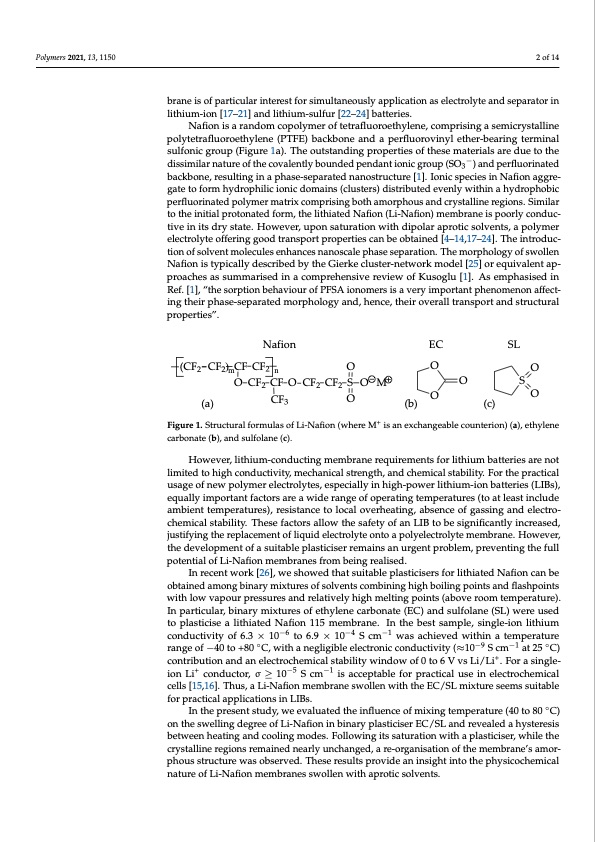
Polymers 2021, 13, x FOR PEER REVIEW 2 of 15 Polymers 2021, 13, 1150 2 of 14 conductivity. Single-ion cationic transfer eliminates the concentration gradient in an elec- trochemical cell along with its problematic consequences [15,16]. At present, the lithium bfroarnme oisf othfepmaretimcublraarnientiesroefstpfaorrtisciumlaurltiannteroeusstlfyorapsipmliucaltaionneoaussellyecatproplyictaetaionndassepelaercattrorlyinte laitnhdiusmep-iaornat[o1r7–in21li]tahniudmli-tihoinum[17-s–u2l1fu]ran[2d2l–i2th4i]ubmat-tseurlifeusr.[22–24]batteries. Nafifioniissaarraannddoomccooppoolylymeerroofftetetrtaraflfuluoororoeeththyylelennee, ,ccoompprrisisininggaasesemicicryrysstatallilninee polytetrafluoroethylene (PTFE) backbone and a perfluorovinyl ether-bearing terminal polytetrafluoroethylene (PTFE) backbone and a perfluorovinyl ether-bearing terminal sul- sulfonic group (Figure 1a). The outstanding properties of these materials are due to the fonic group (Figure 1a). The outstanding properties of these materials are due to the dis- − backbone, resulting in a phase-separated nanostructure [1]. Ionic species in Nafion aggre- backbone, resulting in a phase-separated nanostructure [1]. Ionic species in Nafion aggre- dissimilar nature of the covalently bounded pendant ionic group (SO −) and perfluorinated similar nature of the covalently bounded pendant ionic group (SO33 ) and perfluorinated gate to form hydrophilic ionic domains (clusters) distributed evenly within a hydrophobic gate to form hydrophilic ionic domains (clusters) distributed evenly within a hydrophobic perfluorinated polymer matrix comprising both amorphous and crystalline regions. Similar perfluorinated polymer matrix comprising both amorphous and crystalline regions. Sim- to the initial protonated form, the lithiated Nafion (Li-Nafion) membrane is poorly conduc- ilar to the initial protonated form, the lithiated Nafion (Li-Nafion) membrane is poorly tive in its dry state. However, upon saturation with dipolar aprotic solvents, a polymer conductive in its dry state. However, upon saturation with dipolar aprotic solvents, a pol- electrolyte offering good transport properties can be obtained [4–14,17–24]. The introduc- ymer electrolyte offering good transport properties can be obtained [4–14,17–24]. The in- tion of solvent molecules enhances nanoscale phase separation. The morphology of swollen troduction of solvent molecules enhances nanoscale phase separation. The morphology of Nafion is typically described by the Gierke cluster-network model [25] or equivalent ap- swollen Nafion is typically described by the Gierke cluster-network model [25] or equiv- proaches as summarised in a comprehensive review of Kusoglu [1]. As emphasised in alent approaches as summarised in a comprehensive review of Kusoglu [1]. As empha- Ref. [1], “the sorption behaviour of PFSA ionomers is a very important phenomenon affect- sised in Ref. [1], “the sorption behaviour of PFSA ionomers is a very important phenom- ing their phase-separated morphology and, hence, their overall transport and structural enon affecting their phase-separated morphology and, hence, their overall transport and properties”. structural properties”. Nafion (CF CF)CFCF 22m2n EC SL O O O O CF2 CF O CF2 CF2 S O M (a) CF3 O + carbonate (b), and sulfolane (c). carbonate (b), and sulfolane (c). However,,liltithhiuium--cconduccttiing meembrrane rreequiirreementtss fforrliltithhiiumbbaattteerriieessaarreennoot t lliimiitteedttohiighconduccttiiviitty,,meecchhaannicicaallssttrreennggthth,,aannddcchheemicicaallssttaabbiliiltiyty. .FFoorrththeepprraacctitcicaall ussageoffneewpoollyymeerreelleeccttrroollyytteess,,eesspeecciiaalllyiinhhiiggh--poweerrlliitthiium--iionbaattterriiess((LIIBss)),, eequalllyiimporrttanttffaccttorrssarreeaawiideerraanggeeooffooppeerraattininggtteempeerraatturreess((ttooaattleleaassttininccluluddee aambiientt ttemperattures),, resiisttance tto llocall overheattiing,, absence of gassiing and ellecttro-- cchemiicall ssttabiilliitty. . These ffacttors alllow tthe saffetty off an LIIB tto bbee ssiiggniifificcaanttlly iinccrreeaassed,, O FFiiggurree11..SttrrucctturrallfformullasofLii-Nafifion(whereM isanexchangeabllecountteerriion))((aa)),,eetthyylleennee justifying the replacement of liquid electrolyte onto a polyelectrolyte membrane. However, justifying the replacement of liquid electrolyte onto a polyelectrolyte membrane. How- the development of a suitable plasticiser remains an urgent problem, preventing the full ever, the development of a suitable plasticiser remains an urgent problem, preventing the potential of Li-Nafion membranes from being realised. full potential of Li-Nafion membranes from being realised. In recent work [26], we showed that suitable plasticisers for lithiated Nafion can be In recent work [26], we showed that suitable plasticisers for lithiated Nafion can be obtained among binary mixtures of solvents combining high boiling points and flashpoints obtained among binary mixtures of solvents combining high boiling points and flash- with low vapour pressures and relatively high melting points (above room temperature). points with low vapour pressures and relatively high melting points (above room tem- In particular, binary mixtures of ethylene carbonate (EC) and sulfolane (SL) were used perature). In particular, binary mixtures of ethylene carbonate (EC) and sulfolane (SL) to plasticise a lithiated Nafion 115 membrane. In the best sample, single-ion lithium were used to plasticise a lithiated Nafion 115 membrane. In the best sample, single-ion conductivityof6.3×10−6to6−.69×10−4S−4cm−1−w1asachievedwithinatemperature lithium conductivity of 6.3 × 10 to 6.9 × 10 S cm was achieved within a temperature range of −40 to +80 ◦C, with a negligible electronic conductivity (≈10−9 S cm−1 at 25 ◦C) range of −40 to +80 °C, with a negligible electronic conductivity (≈10−9 S cm−1 at 25 °C) contribution and an electrochemical stability window of 0 to 6 V vs Li/Li+. For a single- contribution and an electrochemical stability window of 0 to 6 V vs Li/Li+. For a single-ion ion Li+ conductor, σ ≥ 10−5 S cm−1 is acceptable for practical use in electrochemical Li+ conductor, σ ≥ 10−5 S cm−1 is acceptable for practical use in electrochemical cells [15,16]. cells [15,16]. Thus, a Li-Nafion membrane swollen with the EC/SL mixture seems suitable Thus, a Li-Nafion membrane swollen with the EC/SL mixture seems suitable for practical C) for practical applications in LIBs. applications in LIBs. ◦ In the present study, we evaluated the influence of mixing temperature (40 to 80 In the present study, we evaluated the influence of mixing temperature (40 to 80 °C) on the swelling degree of Li-Nafion in binary plasticiser EC/SL and revealed a hysteresis on the swelling degree of Li-Nafion in binary plasticiser EC/SL and revealed a hysteresis between heating and cooling modes. Following its saturation with a plasticiser, while the between heating and cooling modes. Following its saturation with a plasticiser, while the crystalline regions remained nearly unchanged, a re-organisation of the membrane’s amor- crystalline regions remained nearly unchanged, a re-organisation of the membrane’s phous structure was observed. These results provide an insight into the physicochemical nature of Li-Nafion membranes swollen with aprotic solvents. (b) O (c) OSPDF Image | Li-Nafion Membrane Plasticised with Ethylene Carbonate Sulfolane
PDF Search Title:
Li-Nafion Membrane Plasticised with Ethylene Carbonate SulfolaneOriginal File Name Searched:
polymers-13-01150.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |