
PDF Publication Title:
Text from PDF Page: 122
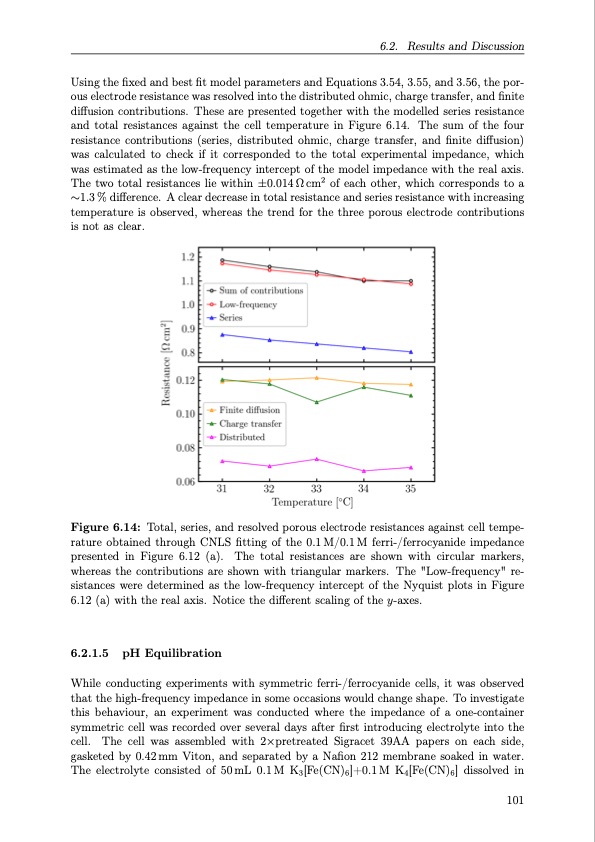
Using the fixed and best fit model parameters and Equations 3.54, 3.55, and 3.56, the por- ous electrode resistance was resolved into the distributed ohmic, charge transfer, and finite diffusion contributions. These are presented together with the modelled series resistance and total resistances against the cell temperature in Figure 6.14. The sum of the four resistance contributions (series, distributed ohmic, charge transfer, and finite diffusion) was calculated to check if it corresponded to the total experimental impedance, which was estimated as the low-frequency intercept of the model impedance with the real axis. The two total resistances lie within ±0.014Ωcm2 of each other, which corresponds to a ∼1.3 % difference. A clear decrease in total resistance and series resistance with increasing temperature is observed, whereas the trend for the three porous electrode contributions is not as clear. Figure 6.14: Total, series, and resolved porous electrode resistances against cell tempe- rature obtained through CNLS fitting of the 0.1M/0.1M ferri-/ferrocyanide impedance presented in Figure 6.12 (a). The total resistances are shown with circular markers, whereas the contributions are shown with triangular markers. The "Low-frequency" re- sistances were determined as the low-frequency intercept of the Nyquist plots in Figure 6.12 (a) with the real axis. Notice the different scaling of the y-axes. 6.2.1.5 pH Equilibration While conducting experiments with symmetric ferri-/ferrocyanide cells, it was observed that the high-frequency impedance in some occasions would change shape. To investigate this behaviour, an experiment was conducted where the impedance of a one-container symmetric cell was recorded over several days after first introducing electrolyte into the cell. The cell was assembled with 2×pretreated Sigracet 39AA papers on each side, gasketed by 0.42 mm Viton, and separated by a Nafion 212 membrane soaked in water. The electrolyte consisted of 50mL 0.1M K3[Fe(CN)6]+0.1M K4[Fe(CN)6] dissolved in 6.2. Results and Discussion 101PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |