
PDF Publication Title:
Text from PDF Page: 037
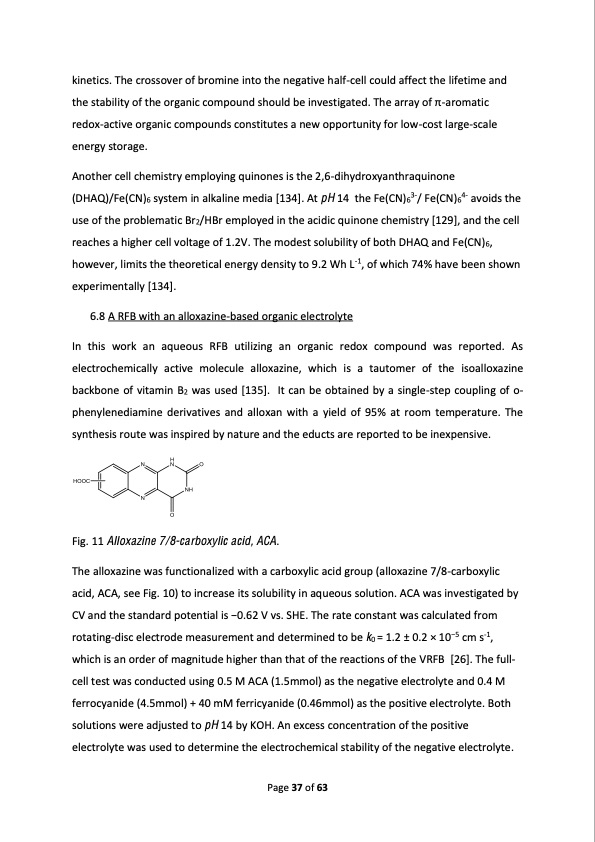
kinetics. The crossover of bromine into the negative half-cell could affect the lifetime and the ��������� �� ��� ������� �������� ������ �� ������������� ��� ����� �� �-aromatic redox-active organic compounds constitutes a new opportunity for low-cost large-scale energy storage. Another cell chemistry employing quinones is the 2,6-dihydroxyanthraquinone (DHAQ)/Fe(CN)6 system in alkaline media [134]. At pH 14 the Fe(CN)63-/ Fe(CN)64- avoids the use of the problematic Br2/HBr employed in the acidic quinone chemistry [129], and the cell reaches a higher cell voltage of 1.2V. The modest solubility of both DHAQ and Fe(CN)6, however, limits the theoretical energy density to 9.2 Wh L-1, of which 74% have been shown experimentally [134]. 6.8 A RFB with an alloxazine-based organic electrolyte In this work an aqueous RFB utilizing an organic redox compound was reported. As electrochemically active molecule alloxazine, which is a tautomer of the isoalloxazine backbone of vitamin B2 was used [135]. It can be obtained by a single-step coupling of o- phenylenediamine derivatives and alloxan with a yield of 95% at room temperature. The synthesis route was inspired by nature and the educts are reported to be inexpensive. Fig. 11 Alloxazine 7/8-carboxylic acid, ACA. The alloxazine was functionalized with a carboxylic acid group (alloxazine 7/8-carboxylic acid, ACA, see Fig. 10) to increase its solubility in aqueous solution. ACA was investigated by CV and the standard potential �� ����� � ��� SHE. The rate constant was calculated from rotating-disc electrode measurement and determined to be k0 = 1.2 ± 0.2 × 10�5 cm s-1, which is an order of magnitude higher than that of the reactions of the VRFB [26]. The full- cell test was conducted using 0.5 M ACA (1.5mmol) as the negative electrolyte and 0.4 M ferrocyanide (4.5mmol) + 40 mM ferricyanide (0.46mmol) as the positive electrolyte. Both solutions were adjusted to pH 14 by KOH. An excess concentration of the positive electrolyte was used to determine the electrochemical stability of the negative electrolyte. Page 37 of 63PDF Image | Redox Flow Batteries Concepts Chemistries
PDF Search Title:
Redox Flow Batteries Concepts ChemistriesOriginal File Name Searched:
5870EAF5-2D70-44C8-A0A7-62D3A1462269.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |