
PDF Publication Title:
Text from PDF Page: 003
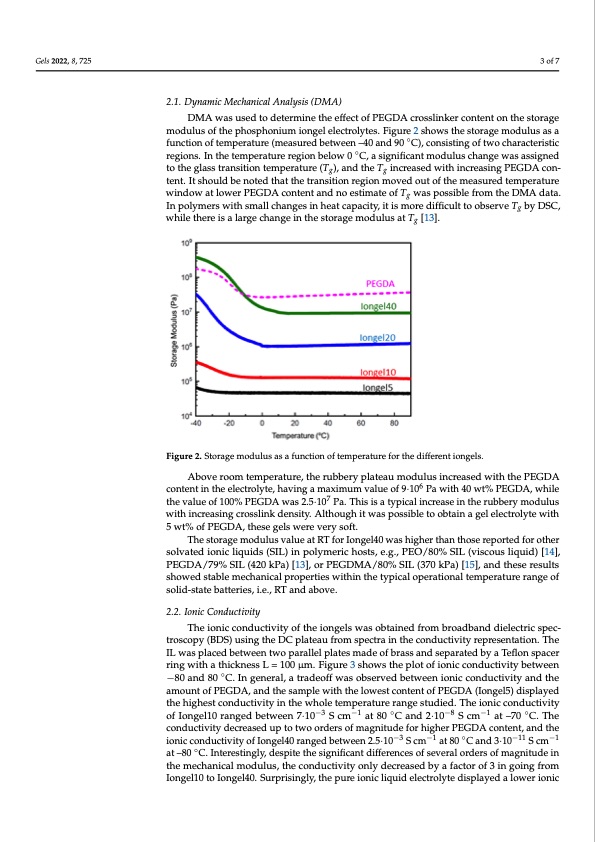
Gels 2022, 8, 725 3 of 7 Figure 1. Synthetic representation of photopolymerization for the obtention of iongels. 2.1. Dynamic Mechanical Analysis (DMA) 2.1. Dynamic Mechanical Analysis (DMA) DMA was used to determine the effect of PEGDA crosslinker content on the storage DMA was used to determine the effect of PEGDA crosslinker content on the storage modulus of the phosphonium iongel electrolytes. Figure 2 shows the storage modulus as modulus of the phosphonium iongel electrolytes. Figure 2 shows the storage modulus as a a function of temperature (measured between –40 and ◦90 °C), consisting of two character- function of temperature (measured between –40 and 90 C), consisting of two characteristic istic regions. In the temperature region be◦low 0 °C, a significant modulus change was regions. In the temperature region below 0 C, a significant modulus change was assigned assigned to the glass transition temperature (Tg), and the Tg increased with increasing to the glass transition temperature (Tg), and the Tg increased with increasing PEGDA con- PEGDA content. It should be noted that the transition region moved out of the measured tent. It should be noted that the transition region moved out of the measured temperature temperature window at lower PEGDA content and no estimate of Tg was possible from window at lower PEGDA content and no estimate of Tg was possible from the DMA data. the DMA data. In polymers with small changes in heat capacity, it is more difficult to In polymers with small changes in heat capacity, it is more difficult to observe Tg by DSC, observe Tg by DSC, while there is a large change in the storage modulus at Tg [13]. while there is a large change in the storage modulus at Tg [13]. Figure 2. Storage modulus as a function of temperature for the different iongels. Figure 2. Storage modulus as a function of temperature for the different iongels. Above room temperature, the rubbery plateau modulus increased with the PEGDA Above room temperature, the rubbery plateau modulus increased with the PEGDA 6 content in the electrolyte, having a maximum value of 9·10 6 Pa with 40 wt% PEGDA, while contentintheelectrolyte,havingamaximumvalueof9·10 Pawith40wt%PEGDA,while 7 the value of 100% PEGDA was 2.5·10 7 Pa. This is a typical increase in the rubbery modulus thevalueof100%PEGDAwas2.5·10 Pa.Thisisatypicalincreaseintherubberymodulus with increasing crosslink density. Although it was possible to obtain a gel electrolyte with with increasing crosslink density. Although it was possible to obtain a gel electrolyte with 5 wt% of PEGDA, these gels were very soft. 5 wt% of PEGDA, these gels were very soft. The storage modulus value at RT for Iongel40 was higher than those reported for other The storage modulus value at RT for Iongel40 was higher than those reported for solvated ionic liquids (SIL) in polymeric hosts, e.g., PEO/80% SIL (viscous liquid) [14], other solvated ionic liquids (SIL) in polymeric hosts, e.g., PEO/80% SIL (viscous liquid) PEGDA/79% SIL (420 kPa) [13], or PEGDMA/80% SIL (370 kPa) [15], and these results showed stable mechanical properties within the typical operational temperature range of solid-state batteries, i.e., RT and above. 2.2. Ionic Conductivity The ionic conductivity of the iongels was obtained from broadband dielectric spec- troscopy (BDS) using the DC plateau from spectra in the conductivity representation. The IL was placed between two parallel plates made of brass and separated by a Teflon spacer ring with a thickness L = 100 μm. Figure 3 shows the plot of ionic conductivity between −80 and 80 ◦C. In general, a tradeoff was observed between ionic conductivity and the amount of PEGDA, and the sample with the lowest content of PEGDA (Iongel5) displayed the highest conductivity in the whole temperature range studied. The ionic conductivity of Iongel10 ranged between 7·10−3 S cm−1 at 80 ◦C and 2·10−8 S cm−1 at –70 ◦C. The conductivity decreased up to two orders of magnitude for higher PEGDA content, and the ionic conductivity of Iongel40 ranged between 2.5·10−3 S cm−1 at 80 ◦C and 3·10−11 S cm−1 at –80 ◦C. Interestingly, despite the significant differences of several orders of magnitude in the mechanical modulus, the conductivity only decreased by a factor of 3 in going from Iongel10 to Iongel40. Surprisingly, the pure ionic liquid electrolyte displayed a lower ionicPDF Image | Phosphonium Iongels for Solid-State Sodium Metal Batteries
PDF Search Title:
Phosphonium Iongels for Solid-State Sodium Metal BatteriesOriginal File Name Searched:
gels-08-00725.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |