
PDF Publication Title:
Text from PDF Page: 003
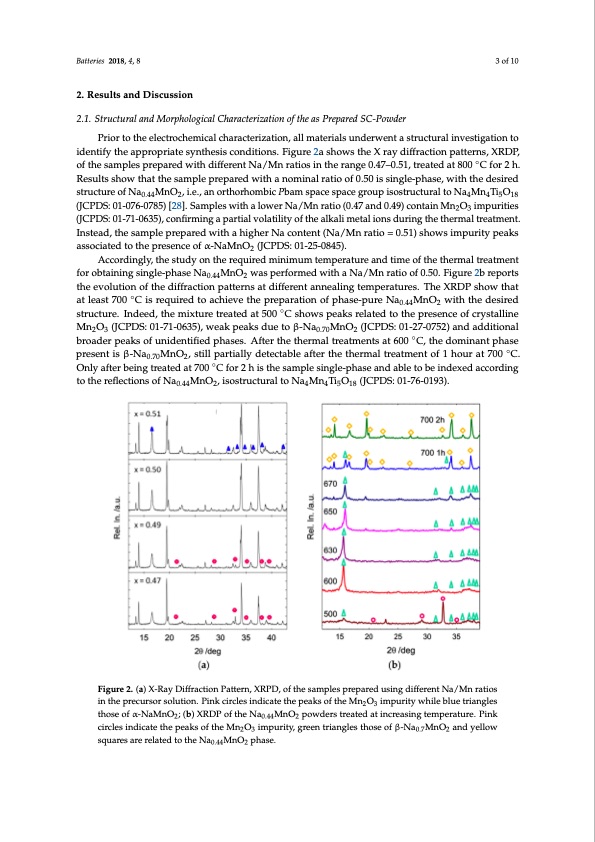
Batteries 2018, 4, 8 3 of 10 2. Results and Discussion 2.1. Structural and Morphological Characterization of the as Prepared SC-Powder Prior to the electrochemical characterization, all materials underwent a structural investigation to identify the appropriate synthesis conditions. Figure 2a shows the X ray diffraction patterns, XRDP, of the samples prepared with different Na/Mn ratios in the range 0.47–0.51, treated at 800 ◦C for 2 h. Results show that the sample prepared with a nominal ratio of 0.50 is single-phase, with the desired structure of Na0.44MnO2, i.e., an orthorhombic Pbam space space group isostructural to Na4Mn4Ti5O18 (JCPDS: 01-076-0785) [28]. Samples with a lower Na/Mn ratio (0.47 and 0.49) contain Mn2O3 impurities (JCPDS: 01-71-0635), confirming a partial volatility of the alkali metal ions during the thermal treatment. Instead, the sample prepared with a higher Na content (Na/Mn ratio = 0.51) shows impurity peaks associated to the presence of α-NaMnO2 (JCPDS: 01-25-0845). Accordingly, the study on the required minimum temperature and time of the thermal treatment for obtaining single-phase Na0.44MnO2 was performed with a Na/Mn ratio of 0.50. Figure 2b reports the evolution of the diffraction patterns at different annealing temperatures. The XRDP show that at least 700 ◦C is required to achieve the preparation of phase-pure Na0.44MnO2 with the desired structure. Indeed, the mixture treated at 500 ◦C shows peaks related to the presence of crystalline Mn2O3 (JCPDS: 01-71-0635), weak peaks due to β-Na0.70MnO2 (JCPDS: 01-27-0752) and additional broader peaks of unidentified phases. After the thermal treatments at 600 ◦C, the dominant phase present is β-Na0.70MnO2, still partially detectable after the thermal treatment of 1 hour at 700 ◦C. Only after being treated at 700 ◦C for 2 h is the sample single-phase and able to be indexed according to the reflections of Na0.44MnO2, isostructural to Na4Mn4Ti5O18 (JCPDS: 01-76-0193). Figure 2. (a) X-Ray Diffraction Pattern, XRPD, of the samples prepared using different Na/Mn ratios in the precursor solution. Pink circles indicate the peaks of the Mn2O3 impurity while blue triangles those of α-NaMnO2; (b) XRDP of the Na0.44MnO2 powders treated at increasing temperature. Pink circles indicate the peaks of the Mn2O3 impurity, green triangles those of β-Na0.7MnO2 and yellow squares are related to the Na0.44MnO2 phase.PDF Image | Sodium-Ion Batteries Obtained through Urea Based
PDF Search Title:
Sodium-Ion Batteries Obtained through Urea BasedOriginal File Name Searched:
10281-250332.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |