
PDF Publication Title:
Text from PDF Page: 027
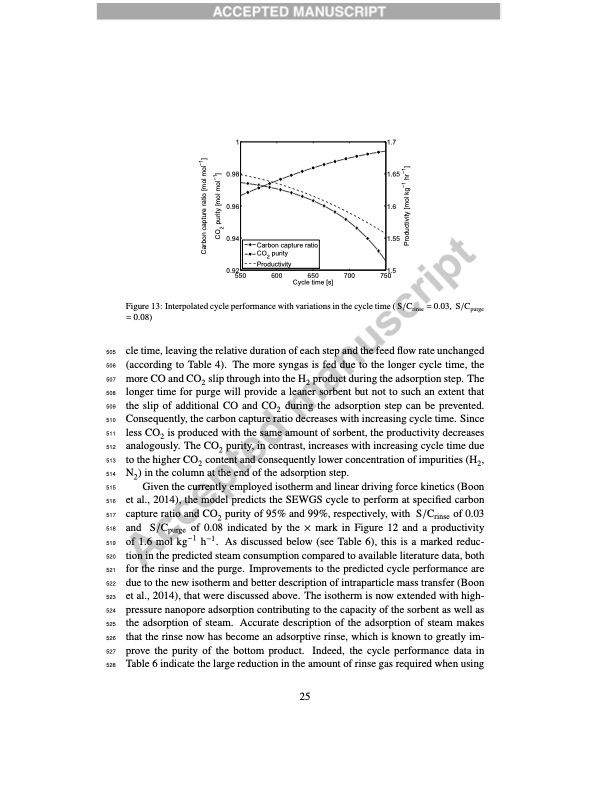
1 0.98 0.96 0.94 0.92 550 1.7 1.65 1.6 1.55 700 750 Carbon capture ratio CO2 purity Productivity 600 650 Cycle time [s] 1.5 Figure 13: Interpolated cycle performance with variations in the cycle time ( S/Crinse = 0.03, S/Cpurge = 0.08) 505 cle time, leaving the relative duration of each step and the feed flow rate unchanged 506 (according to Table 4). The more syngas is fed due to the longer cycle time, the 507 more CO and CO2 slip through into the H2 product during the adsorption step. The 508 longer time for purge will provide a leaner sorbent but not to such an extent that 509 the slip of additional CO and CO2 during the adsorption step can be prevented. 510 Consequently, the carbon capture ratio decreases with increasing cycle time. Since 511 less CO2 is produced with the same amount of sorbent, the productivity decreases 512 analogously. The CO2 purity, in contrast, increases with increasing cycle time due 513 to the higher CO2 content and consequently lower concentration of impurities (H2, 514 N2) in the column at the end of the adsorption step. 515 Given the currently employed isotherm and linear driving force kinetics (Boon 516 et al., 2014), the model predicts the SEWGS cycle to perform at specified carbon 517 capture ratio and CO2 purity of 95% and 99%, respectively, with S/Crinse of 0.03 518 and S/Cpurge of 0.08 indicated by the × mark in Figure 12 and a productivity 519 of 1.6 mol kg−1 h−1. As discussed below (see Table 6), this is a marked reduc- 520 tion in the predicted steam consumption compared to available literature data, both 521 for the rinse and the purge. Improvements to the predicted cycle performance are 522 due to the new isotherm and better description of intraparticle mass transfer (Boon 523 et al., 2014), that were discussed above. The isotherm is now extended with high- 524 pressure nanopore adsorption contributing to the capacity of the sorbent as well as 525 the adsorption of steam. Accurate description of the adsorption of steam makes 526 that the rinse now has become an adsorptive rinse, which is known to greatly im- 527 prove the purity of the bottom product. Indeed, the cycle performance data in 528 Table 6 indicate the large reduction in the amount of rinse gas required when using 25 Carbon capture ratio [mol mol�1] CO2 purity [mol mol�1] Productivity [mol kg�1 hr�1]PDF Image | High-temperature pressure swing adsorption cycle design for sorption
PDF Search Title:
High-temperature pressure swing adsorption cycle design for sorptionOriginal File Name Searched:
high-temperature-pressure-swing-adsorption.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |