
PDF Publication Title:
Text from PDF Page: 046
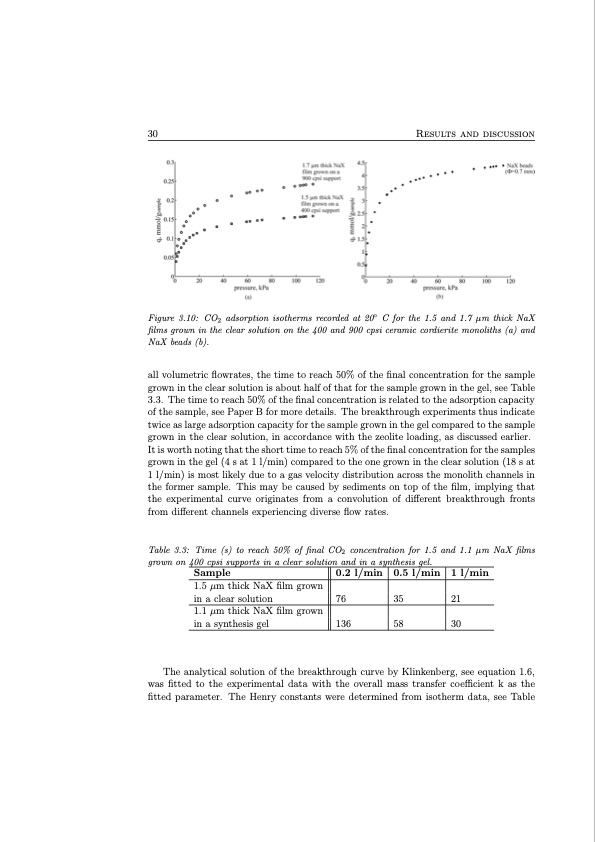
30 Results and discussion Figure 3.10: CO2 adsorption isotherms recorded at 20◦ C for the 1.5 and 1.7 μm thick NaX films grown in the clear solution on the 400 and 900 cpsi ceramic cordierite monoliths (a) and NaX beads (b). all volumetric flowrates, the time to reach 50% of the final concentration for the sample grown in the clear solution is about half of that for the sample grown in the gel, see Table 3.3. The time to reach 50% of the final concentration is related to the adsorption capacity of the sample, see Paper B for more details. The breakthrough experiments thus indicate twice as large adsorption capacity for the sample grown in the gel compared to the sample grown in the clear solution, in accordance with the zeolite loading, as discussed earlier. It is worth noting that the short time to reach 5% of the final concentration for the samples grown in the gel (4 s at 1 l/min) compared to the one grown in the clear solution (18 s at 1 l/min) is most likely due to a gas velocity distribution across the monolith channels in the former sample. This may be caused by sediments on top of the film, implying that the experimental curve originates from a convolution of different breakthrough fronts from different channels experiencing diverse flow rates. Table 3.3: Time (s) to reach 50% of final CO2 concentration for 1.5 and 1.1 μm NaX films grown on 400 cpsi supports in a clear solution and in a synthesis gel. Sample 1.5 μm thick NaX film grown in a clear solution 1.1 μm thick NaX film grown in a synthesis gel 0.2 l/min 76 136 0.5 l/min 35 58 1 l/min 21 30 The analytical solution of the breakthrough curve by Klinkenberg, see equation 1.6, was fitted to the experimental data with the overall mass transfer coefficient k as the fitted parameter. The Henry constants were determined from isotherm data, see TablePDF Image | Structured Zeolite Adsorbents for PSA Applications
PDF Search Title:
Structured Zeolite Adsorbents for PSA ApplicationsOriginal File Name Searched:
structured-zeolites.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |