
PDF Publication Title:
Text from PDF Page: 070
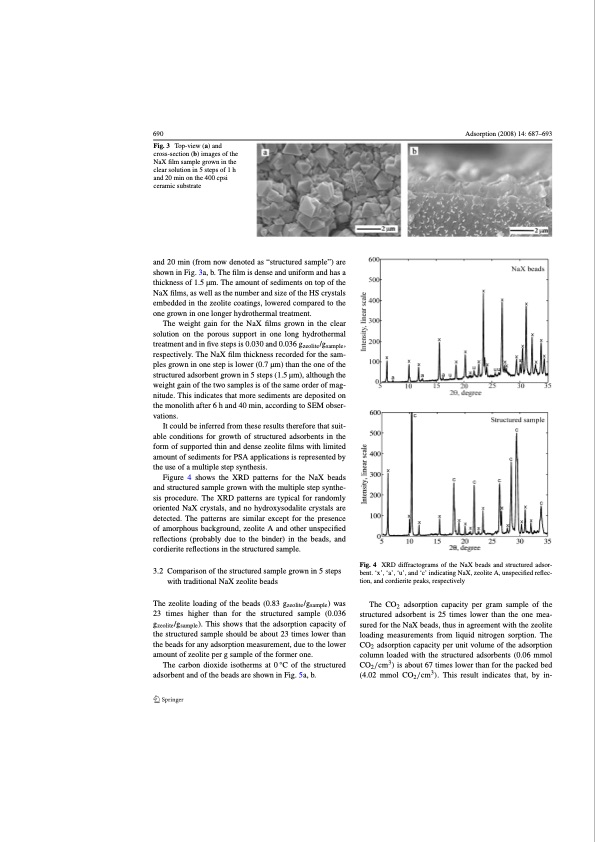
690 Fig. 3 Top-view (a) and cross-section (b) images of the NaX film sample grown in the clear solution in 5 steps of 1 h and 20 min on the 400 cpsi ceramic substrate and 20 min (from now denoted as “structured sample”) are shown in Fig. 3a, b. The film is dense and uniform and has a thickness of 1.5 μm. The amount of sediments on top of the NaX films, as well as the number and size of the HS crystals embedded in the zeolite coatings, lowered compared to the one grown in one longer hydrothermal treatment. The weight gain for the NaX films grown in the clear solution on the porous support in one long hydrothermal treatment and in five steps is 0.030 and 0.036 gzeolite/gsample, respectively. The NaX film thickness recorded for the sam- ples grown in one step is lower (0.7 μm) than the one of the structured adsorbent grown in 5 steps (1.5 μm), although the weight gain of the two samples is of the same order of mag- nitude. This indicates that more sediments are deposited on the monolith after 6 h and 40 min, according to SEM obser- vations. It could be inferred from these results therefore that suit- able conditions for growth of structured adsorbents in the form of supported thin and dense zeolite films with limited amount of sediments for PSA applications is represented by the use of a multiple step synthesis. Figure 4 shows the XRD patterns for the NaX beads and structured sample grown with the multiple step synthe- sis procedure. The XRD patterns are typical for randomly oriented NaX crystals, and no hydroxysodalite crystals are detected. The patterns are similar except for the presence of amorphous background, zeolite A and other unspecified reflections (probably due to the binder) in the beads, and cordierite reflections in the structured sample. 3.2 Comparison of the structured sample grown in 5 steps with traditional NaX zeolite beads The zeolite loading of the beads (0.83 gzeolite /gsample ) was 23 times higher than for the structured sample (0.036 gzeolite /gsample ). This shows that the adsorption capacity of the structured sample should be about 23 times lower than the beads for any adsorption measurement, due to the lower amount of zeolite per g sample of the former one. The carbon dioxide isotherms at 0 °C of the structured adsorbent and of the beads are shown in Fig. 5a, b. Adsorption (2008) 14: 687–693 Fig. 4 XRD diffractograms of the NaX beads and structured adsor- bent. ‘x’, ‘a’, ‘u’, and ‘c’ indicating NaX, zeolite A, unspecified reflec- tion, and cordierite peaks, respectively The CO2 adsorption capacity per gram sample of the structured adsorbent is 25 times lower than the one mea- sured for the NaX beads, thus in agreement with the zeolite loading measurements from liquid nitrogen sorption. The CO2 adsorption capacity per unit volume of the adsorption column loaded with the structured adsorbents (0.06 mmol CO2/cm3) is about 67 times lower than for the packed bed (4.02 mmol CO2 /cm3 ). This result indicates that, by in-PDF Image | Structured Zeolite Adsorbents for PSA Applications
PDF Search Title:
Structured Zeolite Adsorbents for PSA ApplicationsOriginal File Name Searched:
structured-zeolites.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |