
PDF Publication Title:
Text from PDF Page: 071
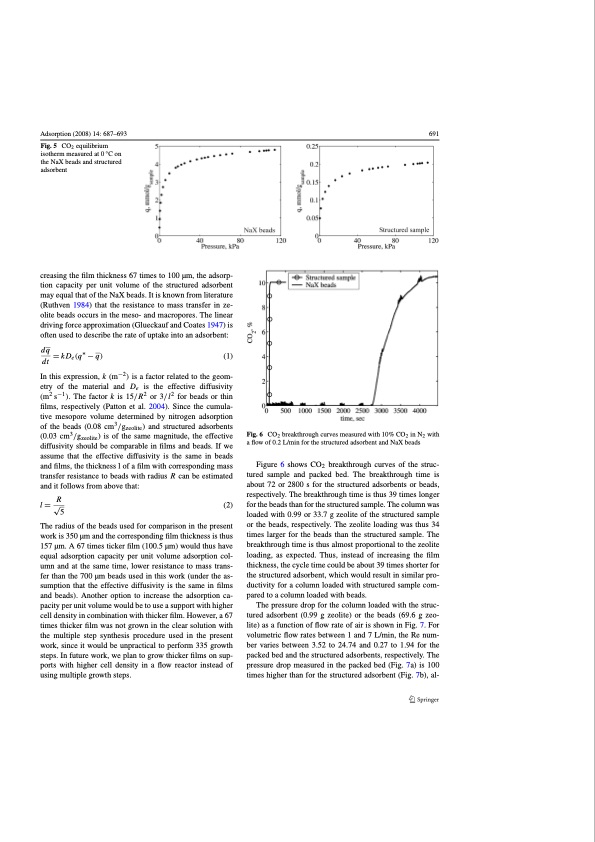
Adsorption (2008) 14: 687–693 Fig. 5 CO2 equilibrium isotherm measured at 0 °C on the NaX beads and structured adsorbent creasing the film thickness 67 times to 100 μm, the adsorp- tion capacity per unit volume of the structured adsorbent may equal that of the NaX beads. It is known from literature (Ruthven 1984) that the resistance to mass transfer in ze- olite beads occurs in the meso- and macropores. The linear driving force approximation (Glueckauf and Coates 1947) is often used to describe the rate of uptake into an adsorbent: dq =kDe(q∗ −q) (1) dt In this expression, k (m−2) is a factor related to the geom- etry of the material and De is the effective diffusivity (m2 s−1). The factor k is 15/R2 or 3/l2 for beads or thin films, respectively (Patton et al. 2004). Since the cumula- tive mesopore volume determined by nitrogen adsorption of the beads (0.08 cm3/gzeolite) and structured adsorbents (0.03 cm3/gzeolite) is of the same magnitude, the effective diffusivity should be comparable in films and beads. If we assume that the effective diffusivity is the same in beads and films, the thickness l of a film with corresponding mass transfer resistance to beads with radius R can be estimated and it follows from above that: R l=√ (2) 5 The radius of the beads used for comparison in the present work is 350 μm and the corresponding film thickness is thus 157 μm. A 67 times ticker film (100.5 μm) would thus have equal adsorption capacity per unit volume adsorption col- umn and at the same time, lower resistance to mass trans- fer than the 700 μm beads used in this work (under the as- sumption that the effective diffusivity is the same in films and beads). Another option to increase the adsorption ca- pacity per unit volume would be to use a support with higher cell density in combination with thicker film. However, a 67 times thicker film was not grown in the clear solution with the multiple step synthesis procedure used in the present work, since it would be unpractical to perform 335 growth steps. In future work, we plan to grow thicker films on sup- ports with higher cell density in a flow reactor instead of using multiple growth steps. 691 CO2 breakthrough curves measured with 10% CO2 in N2 with a flow of 0.2 L/min for the structured adsorbent and NaX beads Figure 6 shows CO2 breakthrough curves of the struc- tured sample and packed bed. The breakthrough time is about 72 or 2800 s for the structured adsorbents or beads, respectively. The breakthrough time is thus 39 times longer for the beads than for the structured sample. The column was loaded with 0.99 or 33.7 g zeolite of the structured sample or the beads, respectively. The zeolite loading was thus 34 times larger for the beads than the structured sample. The breakthrough time is thus almost proportional to the zeolite loading, as expected. Thus, instead of increasing the film thickness, the cycle time could be about 39 times shorter for the structured adsorbent, which would result in similar pro- ductivity for a column loaded with structured sample com- pared to a column loaded with beads. The pressure drop for the column loaded with the struc- tured adsorbent (0.99 g zeolite) or the beads (69.6 g zeo- lite) as a function of flow rate of air is shown in Fig. 7. For volumetric flow rates between 1 and 7 L/min, the Re num- ber varies between 3.52 to 24.74 and 0.27 to 1.94 for the packed bed and the structured adsorbents, respectively. The pressure drop measured in the packed bed (Fig. 7a) is 100 times higher than for the structured adsorbent (Fig. 7b), al- Fig. 6PDF Image | Structured Zeolite Adsorbents for PSA Applications
PDF Search Title:
Structured Zeolite Adsorbents for PSA ApplicationsOriginal File Name Searched:
structured-zeolites.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |