
PDF Publication Title:
Text from PDF Page: 124
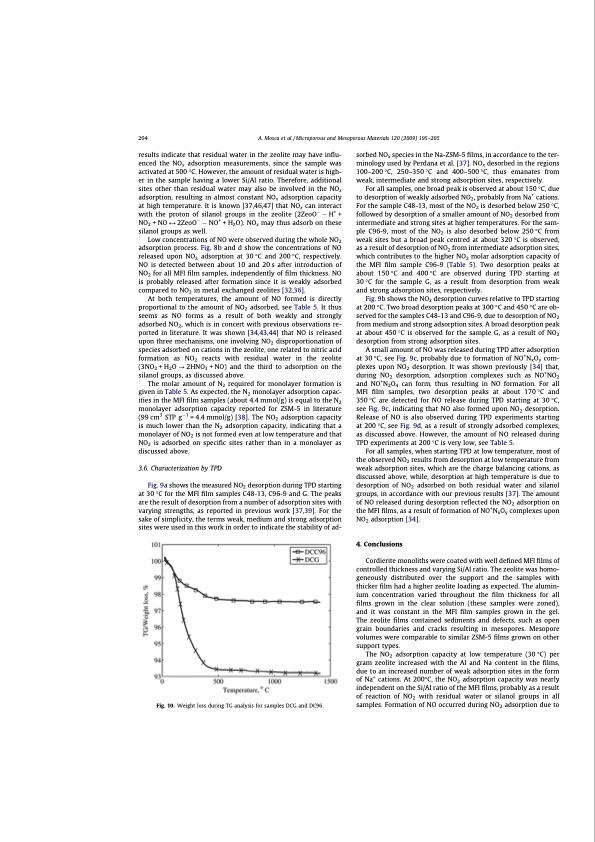
204 A. Mosca et al. / Microporous and Mesoporous Materials 120 (2009) 195–205 results indicate that residual water in the zeolite may have influ- enced the NOx adsorption measurements, since the sample was activated at 500 °C. However, the amount of residual water is high- er in the sample having a lower Si/Al ratio. Therefore, additional sites other than residual water may also be involved in the NOx adsorption, resulting in almost constant NOx adsorption capacity at high temperature. It is known [37,46,47] that NOx can interact with the proton of silanol groups in the zeolite (2ZeoO H+ + NO2 + NO M 2ZeoO NO+ + H2O). NOx may thus adsorb on these silanol groups as well. Low concentrations of NO were observed during the whole NO2 adsorption process. Fig. 8b and d show the concentrations of NO released upon NO2 adsorption at 30 °C and 200 °C, respectively. NO is detected between about 10 and 20 s after introduction of NO2 for all MFI film samples, independently of film thickness. NO is probably released after formation since it is weakly adsorbed compared to NO2 in metal exchanged zeolites [32,36]. At both temperatures, the amount of NO formed is directly proportional to the amount of NO2 adsorbed, see Table 5. It thus seems as NO forms as a result of both weakly and strongly adsorbed NO2, which is in concert with previous observations re- ported in literature. It was shown [34,43,44] that NO is released upon three mechanisms, one involving NO2 disproportionation of species adsorbed on cations in the zeolite, one related to nitric acid formation as NO2 reacts with residual water in the zeolite (3NO2 + H2O ? 2HNO3 + NO) and the third to adsorption on the silanol groups, as discussed above. The molar amount of N2 required for monolayer formation is given in Table 5. As expected, the N2 monolayer adsorption capac- ities in the MFI film samples (about 4.4 mmol/g) is equal to the N2 monolayer adsorption capacity reported for ZSM-5 in literature (99 cm3 STP g1 = 4.4 mmol/g) [38]. The NO2 adsorption capacity is much lower than the N2 adsorption capacity, indicating that a monolayer of NO2 is not formed even at low temperature and that NO2 is adsorbed on specific sites rather than in a monolayer as discussed above. 3.6. Characterization by TPD Fig. 9a shows the measured NO2 desorption during TPD starting at 30 °C for the MFI film samples C48-13, C96-9 and G. The peaks are the result of desorption from a number of adsorption sites with varying strengths, as reported in previous work [37,39]. For the sake of simplicity, the terms weak, medium and strong adsorption sites were used in this work in order to indicate the stability of ad- sorbed NOx species in the Na-ZSM-5 films, in accordance to the ter- minology used by Perdana et al. [37]. NOx desorbed in the regions 100–200 °C, 250–350 °C and 400–500 °C, thus emanates from weak, intermediate and strong adsorption sites, respectively. For all samples, one broad peak is observed at about 150 °C, due to desorption of weakly adsorbed NO2, probably from Na+ cations. For the sample C48-13, most of the NO2 is desorbed below 250 °C, followed by desorption of a smaller amount of NO2 desorbed from intermediate and strong sites at higher temperatures. For the sam- ple C96-9, most of the NO2 is also desorbed below 250 °C from weak sites but a broad peak centred at about 320 °C is observed, as a result of desorption of NO2 from intermediate adsorption sites, which contributes to the higher NO2 molar adsorption capacity of the MFI film sample C96-9 (Table 5). Two desorption peaks at about 150 °C and 400 °C are observed during TPD starting at 30 °C for the sample G, as a result from desorption from weak and strong adsorption sites, respectively. Fig. 9b shows the NO2 desorption curves relative to TPD starting at 200 °C. Two broad desorption peaks at 300 °C and 450 °C are ob- served for the samples C48-13 and C96-9, due to desorption of NO2 from medium and strong adsorption sites. A broad desorption peak at about 450 °C is observed for the sample G, as a result of NO2 desorption from strong adsorption sites. A small amount of NO was released during TPD after adsorption at 30 °C, see Fig. 9c, probably due to formation of NO+NxOy com- plexes upon NO2 desorption. It was shown previously [34] that, during NO2 desorption, adsorption complexes such as NO+NO2 and NO+N2O4 can form, thus resulting in NO formation. For all MFI film samples, two desorption peaks at about 170°C and 350 °C are detected for NO release during TPD starting at 30 °C, see Fig. 9c, indicating that NO also formed upon NO2 desorption. Release of NO is also observed during TPD experiments starting at 200 °C, see Fig. 9d, as a result of strongly adsorbed complexes, as discussed above. However, the amount of NO released during TPD experiments at 200 °C is very low, see Table 5. For all samples, when starting TPD at low temperature, most of the observed NO2 results from desorption at low temperature from weak adsorption sites, which are the charge balancing cations, as discussed above, while, desorption at high temperature is due to desorption of NO2 adsorbed on both residual water and silanol groups, in accordance with our previous results [37]. The amount of NO released during desorption reflected the NO2 adsorption on the MFI films, as a result of formation of NO+NxOy complexes upon NO2 adsorption [34]. 4. Conclusions Cordierite monoliths were coated with well defined MFI films of controlled thickness and varying Si/Al ratio. The zeolite was homo- geneously distributed over the support and the samples with thicker film had a higher zeolite loading as expected. The alumin- ium concentration varied throughout the film thickness for all films grown in the clear solution (these samples were zoned), and it was constant in the MFI film samples grown in the gel. The zeolite films contained sediments and defects, such as open grain boundaries and cracks resulting in mesopores. Mesopore volumes were comparable to similar ZSM-5 films grown on other support types. The NO2 adsorption capacity at low temperature (30 °C) per gram zeolite increased with the Al and Na content in the films, due to an increased number of weak adsorption sites in the form of Na+ cations. At 200°C, the NO2 adsorption capacity was nearly independent on the Si/Al ratio of the MFI films, probably as a result of reaction of NO2 with residual water or silanol groups in all samples. Formation of NO occurred during NO2 adsorption due to Fig. 10. Weight loss during TG analysis for samples DCG and DC96.PDF Image | Structured Zeolite Adsorbents for PSA Applications
PDF Search Title:
Structured Zeolite Adsorbents for PSA ApplicationsOriginal File Name Searched:
structured-zeolites.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |