
PDF Publication Title:
Text from PDF Page: 008
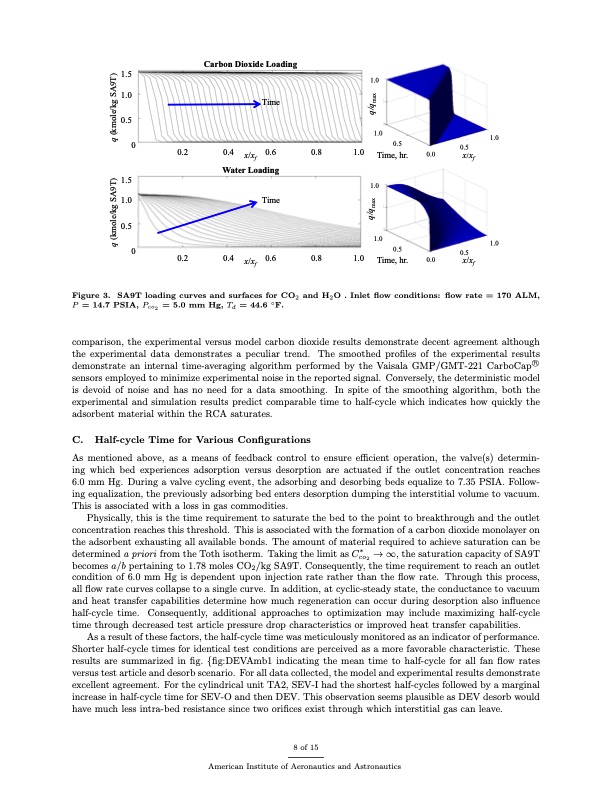
Carbon Dioxide Loading 1.5 1.0 0.5 0 1.5 1.0 Time Water Loading Time 1.0 1.0 0.2 0.8 1.0 0.8 1.0 0.5 0.5 0.0 x/xf 0.4 x/xf 0.6 1.0 Time, hr. 1.0 1.0 0.5 0 0.2 1.0 Time, hr. 0.5 0.5 0.0 x/xf 0.4 x/xf 0.6 Figure 3. SA9T loading curves and surfaces for CO2 and H2O . Inlet flow conditions: flow rate = 170 ALM, P =14.7PSIA,Pco2 =5.0mmHg,Td =44.6◦F. comparison, the experimental versus model carbon dioxide results demonstrate decent agreement although the experimental data demonstrates a peculiar trend. The smoothed profiles of the experimental results demonstrate an internal time-averaging algorithm performed by the Vaisala GMP/GMT-221 CarboCap⃝R sensors employed to minimize experimental noise in the reported signal. Conversely, the deterministic model is devoid of noise and has no need for a data smoothing. In spite of the smoothing algorithm, both the experimental and simulation results predict comparable time to half-cycle which indicates how quickly the adsorbent material within the RCA saturates. C. Half-cycle Time for Various Configurations As mentioned above, as a means of feedback control to ensure efficient operation, the valve(s) determin- ing which bed experiences adsorption versus desorption are actuated if the outlet concentration reaches 6.0 mm Hg. During a valve cycling event, the adsorbing and desorbing beds equalize to 7.35 PSIA. Follow- ing equalization, the previously adsorbing bed enters desorption dumping the interstitial volume to vacuum. This is associated with a loss in gas commodities. Physically, this is the time requirement to saturate the bed to the point to breakthrough and the outlet concentration reaches this threshold. This is associated with the formation of a carbon dioxide monolayer on the adsorbent exhausting all available bonds. The amount of material required to achieve saturation can be determined a priori from the Toth isotherm. Taking the limit as C∗ → ∞, the saturation capacity of SA9T co2 becomes a/b pertaining to 1.78 moles CO2/kg SA9T. Consequently, the time requirement to reach an outlet condition of 6.0 mm Hg is dependent upon injection rate rather than the flow rate. Through this process, all flow rate curves collapse to a single curve. In addition, at cyclic-steady state, the conductance to vacuum and heat transfer capabilities determine how much regeneration can occur during desorption also influence half-cycle time. Consequently, additional approaches to optimization may include maximizing half-cycle time through decreased test article pressure drop characteristics or improved heat transfer capabilities. As a result of these factors, the half-cycle time was meticulously monitored as an indicator of performance. Shorter half-cycle times for identical test conditions are perceived as a more favorable characteristic. These results are summarized in fig. {fig:DEVAmb1 indicating the mean time to half-cycle for all fan flow rates versus test article and desorb scenario. For all data collected, the model and experimental results demonstrate excellent agreement. For the cylindrical unit TA2, SEV-I had the shortest half-cycles followed by a marginal increase in half-cycle time for SEV-O and then DEV. This observation seems plausible as DEV desorb would have much less intra-bed resistance since two orifices exist through which interstitial gas can leave. 8 of 15 American Institute of Aeronautics and Astronautics q (kmole/kg SA9T) q (kmole/kg SA9T) q/qmax q/qmaxPDF Image | Vacuum Swing Adsorption Units for Spacesuit Carbon Dioxide and Humidity Control
PDF Search Title:
Vacuum Swing Adsorption Units for Spacesuit Carbon Dioxide and Humidity ControlOriginal File Name Searched:
10559884.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |