
PDF Publication Title:
Text from PDF Page: 012
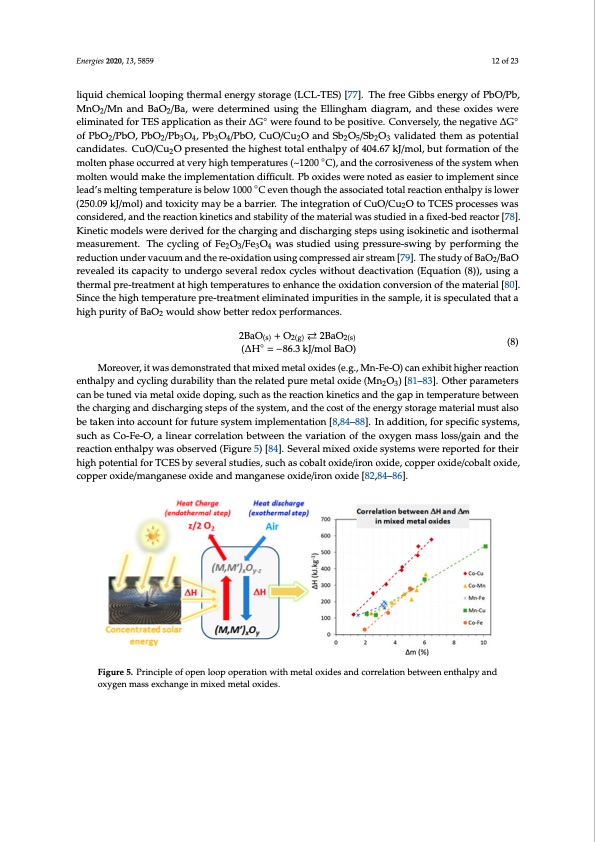
Energies 2020, 13, x FOR PEER REVIEW 11 of 24 conversion rate of BaCO3-BaSiO3 mixture with CaCO3 was about 60%, which closely relates to the expected amount of active material. Energies 2020, 13, 5859 BaO(s) + CO2(g) ⇄ BaCO3(s) 12 of 23 (5) (ΔH° = −272.5 kJ/mol) liquid chemical looping thermal energy storage (LCL-TES) [77]. The free Gibbs energy of PbO/Pb, BaCO3(s) + BaSiO3(s) ⇄ Ba2SiO4(s) + CO2(g) MnO /Mn and BaO /Ba, were determined using the Ellingham diagram, and these oxides were 2 2 (ΔH850 °C = 126.9 kJ/mol) eliminated for TES application as their ∆G◦ were found to be positive. Conversely, the negative ∆G◦ Application of lithium silicate for TCES at high temperatures was proposed by Takasu et al. [73]. of PbO2/PbO, PbO2/Pb3O4, Pb3O4/PbO, CuO/Cu2O and Sb2O5/Sb2O3 validated them as potential The carbonation/decarbonation of the system (Equation (6)) was tested in TGA under various CO2 candidates. CuO/Cu2O presented the highest total enthalpy of 404.67 kJ/mol, but formation of the pressures and presented a gravimetric energy density of 780 kJ/kg at around 400–700 °C, under 100% molten phase occurred at very high temperatures (~1200 ◦C), and the corrosiveness of the system when CO2, with good durability over the course of 5 cycles. molten would make the implementation difficult. Pb oxides were noted as easier to implement since ◦ lead’smeltingtemperatureisbLelio4SwiO140(s0)0+COe2v(ge)n⇄thLoi2uCgOh3t(sh)e+aLsis2oSicOia3t(es)d+tΔotHalrreactionenthalpyislower (6) (250.09 kJ/mol) and toxicity may be a barrie(Δr.HTrh=e−i9n4tekgJr/matoiol)n of CuO/Cu2O to TCES processes was considered, and the reaction kinetics and stability of the material was studied in a fixed-bed reactor [78]. The carbonation of transition metals was also considered to provide new materials for TCES at Kinetic models were derived for the charging and discharging steps using isokinetic and isothermal temperatures below 500 °C [74]. The carbonation of CoO, MnO, PbO and ZnO was studied, to obtain measurement. The cycling of Fe2O3/Fe3O4 was studied using pressure-swing by performing the CoCO3, MnCO3, PbCO3 and ZnCO3, respectively, under high CO2 pressure (8–50 bar), along with the reduction under vacuum and the re-oxidation using compressed air stream [79]. The study of BaO2/BaO effect of moisture and temperature (25–500 °C). Among these, only ZnCO3 could not be obtained. At revealed its capacity to undergo several redox cycles without deactivation (Equation (8)), using a temperatures between 50 and 500 °C, in the presence of moisture under 8 bar CO2, the corresponding thermal pre-treatment at high temperatures to enhance the oxidation conversion of the material [80]. ternary oxides of CoO and MnO were obtained. In the same conditions, PbO reacted to give both Since the high temperature pre-treatment eliminated impurities in the sample, it is speculated that a PbCO3.PbO and PbCO3.2PbO, and the latter was successfully cycled by varying the pressure between high purity of BaO2 would show better redox performances. presence of water. 8 and 2 bar. The carbonation of MnO and PbO was also observed in a reactor, under 50 bar in the 2BaO(s) + O2(g) 2BaO2(s) (∆H◦ = −86.3 kJ/mol BaO) (8) Moreover, it was demonstrated that mixed metal oxides (e.g., Mn-Fe-O) can exhibit higher reaction 4. TCES systems Based on Metal Oxides Metal oxide based TCES systems are especially attractive as they permit working with an open enthalpy and cycling durability than the related pure metal oxide (Mn2O3) [81–83]. Other parameters cycle, using air (Equation (7), Figure 5). For this reason, the study of metal oxide systems in similar can be tuned via metal oxide doping, such as the reaction kinetics and the gap in temperature between conditions with control of oxygen partial pressure (pO2) is important. As a common trend, it can be the charging and discharging steps of the system, and the cost of the energy storage material must also observed that the reduction temperature decreases together with lower partial pressure of the be taken into account for future system implementation [8,84–88]. In addition, for specific systems, reactive gas (O2). The variation of the temperature as a function of pO2 was illustrated using a Van’t such as Co-Fe-O, a linear correlation between the variation of the oxygen mass loss/gain and the Hoff diagram for several metal oxide pairs (Figure 6). reaction enthalpy was observed (Figure 5) [84]. Several mixed oxide systems were reported for their high potential for TCES by several studies, such as cobalt oxide/iron oxide, copper oxide/cobalt oxide, MOred(s) + O2(g) ⇄ MOox(s) + ΔHr copper oxide/manganese oxide and manganese oxide/iron oxide [82,84–86]. (7) (4) Fiiggurree55. .Prriincciiplleoffoopeenloloopooppeerraattiioonwitithmeettaallooxxidideessaannddccoorrreelalattioionnbbeettweeeneenntthhaallpyaannd ooxxygeenmaassseexxcchhaannggeeininmixixeeddmeetatallooxxidideess. .PDF Image | Hi Temp Thermochemical Energy Storage via Solid Gas Reactions
PDF Search Title:
Hi Temp Thermochemical Energy Storage via Solid Gas ReactionsOriginal File Name Searched:
energies-13-05859.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |