
PDF Publication Title:
Text from PDF Page: 013
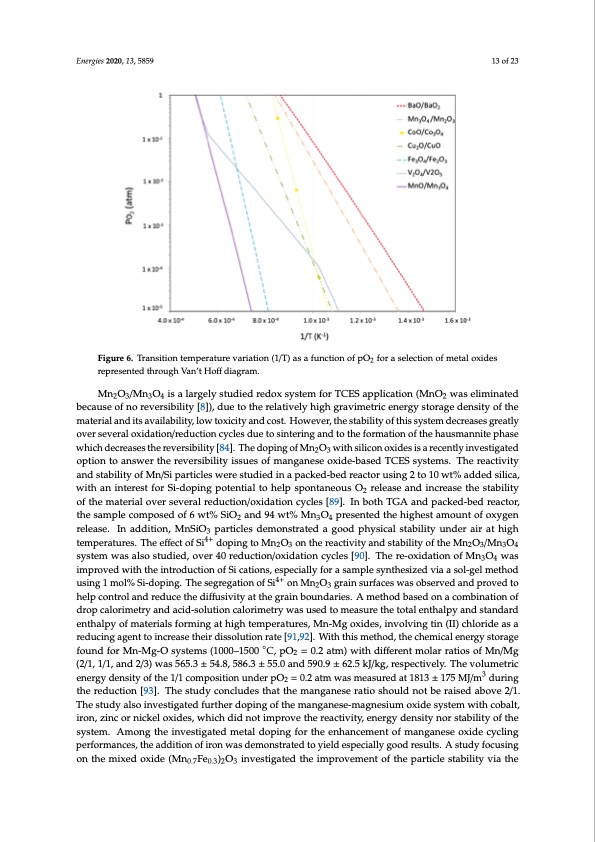
Energies 2020, 13, 5859 13 of 23 Energies 2020, 13, x FOR PEER REVIEW 12 of 24 Figure 6. Transition temperature variation (1/T) as a function of pO2 for a selection of metal oxides Figure 6. Transition temperature variation (1/T) as a function of pO2 for a selection of metal oxides represented through Van’t Hoff diagram. represented through Van’t Hoff diagram. Mn2O3/Mn3O4 is a largely studied redox system for TCES application (MnO2 was eliminated The potential of CuO/Cu2O, Co3O4/CoO, Mn2O3/Mn3O4 and Pb3O4/PbO was investigated under because of no reversibility [8]), due to the relatively high gravimetric energy storage density of the isotherms while varying pO2 between 0.5 and 0.8 bar [75]. The copper and cobalt oxides showed good material and its availability, low toxicity and cost. However, the stability of this system decreases greatly reversibility, but manganese oxide showed a beginning of sintering and lead oxide was eliminated over several oxidation/reduction cycles due to sintering and to the formation of the hausmannite phase as it showed no potential under these operating conditions. The Cu2O/CuO system is also interesting which decreases the reversibility [84]. The doping of Mn2O3 with silicon oxides is a recently investigated as it possesses high reaction enthalpy and reacts at high temperatures [76]. This system was studied option to answer the reversibility issues of manganese oxide-based TCES systems. The reactivity between 800 and 930 °C and focused on the effect of partial pressure variation on the reaction kinetics and stability of Mn/Si particles were studied in a packed-bed reactor using 2 to 10 wt% added silica, with pO2 = 0.1, 0.2, 0.5 and 1.0 bar. The Avrami-Erofeev’s two-dimensional nucleation model (A2) with an interest for Si-doping potential to help spontaneous O2 release and increase the stability was determined as the best fitting conversion model and gave an activation energy of 233 kJ/mol, of the material over several reduction/oxidation cycles [89]. In both TGA and packed-bed reactor, with a frequency factor of 5 × 109 1/s. The potential of liquid multivalent metal oxides was tested in the sample composed of 6 wt% SiO2 and 94 wt% Mn3O4 presented the highest amount of oxygen liquid chemical looping thermal energy storage (LCL-TES) [77]. The free Gibbs energy of PbO/Pb, release. In addition, MnSiO3 particles demonstrated a good physical stability under air at high MnO2/Mn and BaO2/Ba, were determined using the Ellingham diagram, and these oxides were temperatures. The effect of Si4+ doping to Mn2O3 on the reactivity and stability of the Mn2O3/Mn3O4 eliminated for TES application as their ΔG° were found to be positive. Conversely, the negative ΔG° system was also studied, over 40 reduction/oxidation cycles [90]. The re-oxidation of Mn3O4 was of PbO2/PbO, PbO2/Pb3O4, Pb3O4/PbO, CuO/Cu2O and Sb2O5/Sb2O3 validated them as potential improved with the introduction of Si cations, especially for a sample synthesized via a sol-gel method candidates. CuO/Cu2O presented the highest total enthalpy of 404.67 kJ/mol, but formation of the using 1 mol% Si-doping. The segregation of Si4+ on Mn2O3 grain surfaces was observed and proved to molten phase occurred at very high temperatures (~1200 °C), and the corrosiveness of the system help control and reduce the diffusivity at the grain boundaries. A method based on a combination of when molten would make the implementation difficult. Pb oxides were noted as easier to implement drop calorimetry and acid-solution calorimetry was used to measure the total enthalpy and standard since lead’s melting temperature is below 1000 °C even though the associated total reaction enthalpy enthalpy of materials forming at high temperatures, Mn-Mg oxides, involving tin (II) chloride as a is lower (250.09 kJ/mol) and toxicity may be a barrier. The integration of CuO/Cu2O to TCES processes reducing agent to increase their dissolution rate [91,92]. With this method, the chemical energy storage was considered, and the reaction kinetics and stability of the material was studied in a fixed-bed found for Mn-Mg-O systems (1000–1500 ◦C, pO2 = 0.2 atm) with different molar ratios of Mn/Mg reactor [78]. Kinetic models were derived for the charging and discharging steps using isokinetic and (2/1, 1/1, and 2/3) was 565.3 ± 54.8, 586.3 ± 55.0 and 590.9 ± 62.5 kJ/kg, respectively. The volumetric isothermal measurement. The cycling of Fe2O3/Fe3O4 was studied using pressure-swing by energy density of the 1/1 composition under pO2 = 0.2 atm was measured at 1813 ± 175 MJ/m3 during performing the reduction under vacuum and the re-oxidation using compressed air stream [79]. The the reduction [93]. The study concludes that the manganese ratio should not be raised above 2/1. study of BaO2/BaO revealed its capacity to undergo several redox cycles without deactivation The study also investigated further doping of the manganese-magnesium oxide system with cobalt, (Equation (8)), using a thermal pre-treatment at high temperatures to enhance the oxidation iron, zinc or nickel oxides, which did not improve the reactivity, energy density nor stability of the conversion of the material [80]. Since the high temperature pre-treatment eliminated impurities in system. Among the investigated metal doping for the enhancement of manganese oxide cycling the sample, it is speculated that a high purity of BaO2 would show better redox performances. performances, the addition of iron was demonstrated to yield especially good results. A study focusing 2BaO(s) + O2(g) ⇄ 2BaO2(s) on the mixed oxide (Mn0.7Fe0.3)2O3 investigated the improvement of the particle stability via the (ΔH° = −86.3 kJ/mol BaO) (8)PDF Image | Hi Temp Thermochemical Energy Storage via Solid Gas Reactions
PDF Search Title:
Hi Temp Thermochemical Energy Storage via Solid Gas ReactionsOriginal File Name Searched:
energies-13-05859.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |