
PDF Publication Title:
Text from PDF Page: 003
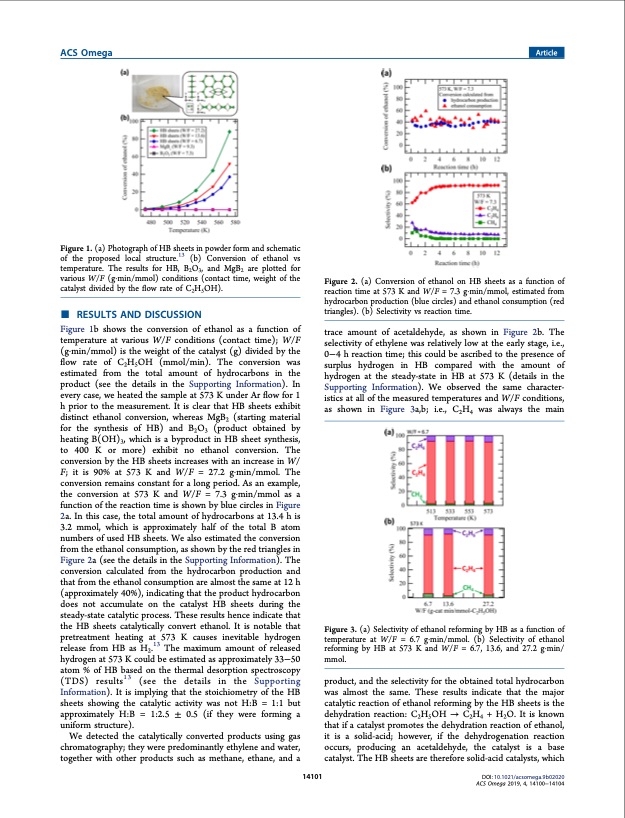
ACS Omega Article Figure 1. (a) Photograph of HB sheets in powder form and schematic of the proposed local structure.13 (b) Conversion of ethanol vs temperature. The results for HB, B2O3, and MgB2 are plotted for various W/F (g·min/mmol) conditions (contact time, weight of the catalyst divided by the flow rate of C2H5OH). ■ RESULTS AND DISCUSSION Figure 1b shows the conversion of ethanol as a function of temperature at various W/F conditions (contact time); W/F (g·min/mmol) is the weight of the catalyst (g) divided by the flow rate of C2H5OH (mmol/min). The conversion was estimated from the total amount of hydrocarbons in the product (see the details in the Supporting Information). In every case, we heated the sample at 573 K under Ar flow for 1 h prior to the measurement. It is clear that HB sheets exhibit distinct ethanol conversion, whereas MgB2 (starting material for the synthesis of HB) and B2O3 (product obtained by heating B(OH)3, which is a byproduct in HB sheet synthesis, to 400 K or more) exhibit no ethanol conversion. The conversion by the HB sheets increases with an increase in W/ F; it is 90% at 573 K and W/F = 27.2 g·min/mmol. The conversion remains constant for a long period. As an example, the conversion at 573 K and W/F = 7.3 g·min/mmol as a function of the reaction time is shown by blue circles in Figure 2a. In this case, the total amount of hydrocarbons at 13.4 h is 3.2 mmol, which is approximately half of the total B atom numbers of used HB sheets. We also estimated the conversion from the ethanol consumption, as shown by the red triangles in Figure 2a (see the details in the Supporting Information). The conversion calculated from the hydrocarbon production and that from the ethanol consumption are almost the same at 12 h (approximately 40%), indicating that the product hydrocarbon does not accumulate on the catalyst HB sheets during the steady-state catalytic process. These results hence indicate that the HB sheets catalytically convert ethanol. It is notable that pretreatment heating at 573 K causes inevitable hydrogen release from HB as H2.13 The maximum amount of released hydrogen at 573 K could be estimated as approximately 33−50 atom % of HB based on the thermal desorption spectroscopy (TDS) results13 (see the details in the Supporting Information). It is implying that the stoichiometry of the HB sheets showing the catalytic activity was not H:B = 1:1 but approximately H:B = 1:2.5 ± 0.5 (if they were forming a uniform structure). We detected the catalytically converted products using gas chromatography; they were predominantly ethylene and water, together with other products such as methane, ethane, and a 14101 Figure 2. (a) Conversion of ethanol on HB sheets as a function of reaction time at 573 K and W/F = 7.3 g·min/mmol, estimated from hydrocarbon production (blue circles) and ethanol consumption (red triangles). (b) Selectivity vs reaction time. trace amount of acetaldehyde, as shown in Figure 2b. The selectivity of ethylene was relatively low at the early stage, i.e., 0−4 h reaction time; this could be ascribed to the presence of surplus hydrogen in HB compared with the amount of hydrogen at the steady-state in HB at 573 K (details in the Supporting Information). We observed the same character- istics at all of the measured temperatures and W/F conditions, as shown in Figure 3a,b; i.e., C2H4 was always the main Figure 3. (a) Selectivity of ethanol reforming by HB as a function of temperature at W/F = 6.7 g·min/mmol. (b) Selectivity of ethanol reforming by HB at 573 K and W/F = 6.7, 13.6, and 27.2 g·min/ mmol. product, and the selectivity for the obtained total hydrocarbon was almost the same. These results indicate that the major catalytic reaction of ethanol reforming by the HB sheets is the dehydration reaction: C2H5OH → C2H4 + H2O. It is known that if a catalyst promotes the dehydration reaction of ethanol, it is a solid-acid; however, if the dehydrogenation reaction occurs, producing an acetaldehyde, the catalyst is a base catalyst. The HB sheets are therefore solid-acid catalysts, which DOI: 10.1021/acsomega.9b02020 ACS Omega 2019, 4, 14100−14104PDF Image | Hydrogenated Borophene Shows Catalytic Activity as Solid Acid
PDF Search Title:
Hydrogenated Borophene Shows Catalytic Activity as Solid AcidOriginal File Name Searched:
Hydrogenated_Borophene_Shows_Catalytic_Activity_as.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |