
PDF Publication Title:
Text from PDF Page: 004
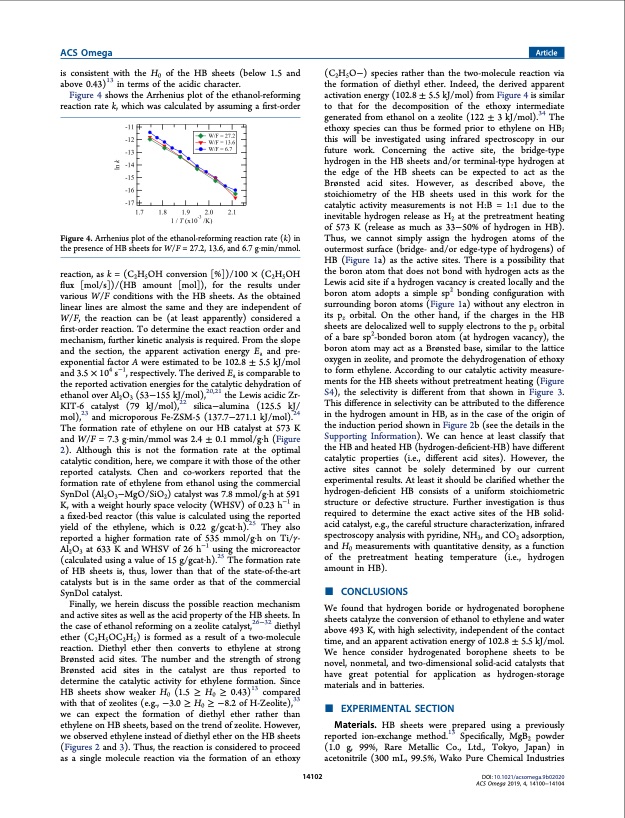
ACS Omega Article is consistent with the H0 of the HB sheets (below 1.5 and above 0.43)13 in terms of the acidic character. Figure 4 shows the Arrhenius plot of the ethanol-reforming reaction rate k, which was calculated by assuming a first-order (C2H5O−) species rather than the two-molecule reaction via the formation of diethyl ether. Indeed, the derived apparent activation energy (102.8 ± 5.5 kJ/mol) from Figure 4 is similar to that for the decomposition of the ethoxy intermediate generated from ethanol on a zeolite (122 ± 3 kJ/mol).34 The ethoxy species can thus be formed prior to ethylene on HB; this will be investigated using infrared spectroscopy in our future work. Concerning the active site, the bridge-type hydrogen in the HB sheets and/or terminal-type hydrogen at the edge of the HB sheets can be expected to act as the Brønsted acid sites. However, as described above, the stoichiometry of the HB sheets used in this work for the catalytic activity measurements is not H:B = 1:1 due to the inevitable hydrogen release as H2 at the pretreatment heating of 573 K (release as much as 33−50% of hydrogen in HB). Thus, we cannot simply assign the hydrogen atoms of the outermost surface (bridge- and/or edge-type of hydrogens) of HB (Figure 1a) as the active sites. There is a possibility that the boron atom that does not bond with hydrogen acts as the Lewis acid site if a hydrogen vacancy is created locally and the boron atom adopts a simple sp2 bonding configuration with surrounding boron atoms (Figure 1a) without any electron in its pz orbital. On the other hand, if the charges in the HB sheets are delocalized well to supply electrons to the pz orbital of a bare sp2-bonded boron atom (at hydrogen vacancy), the boron atom may act as a Brønsted base, similar to the lattice oxygen in zeolite, and promote the dehydrogenation of ethoxy to form ethylene. According to our catalytic activity measure- ments for the HB sheets without pretreatment heating (Figure S4), the selectivity is different from that shown in Figure 3. This difference in selectivity can be attributed to the difference in the hydrogen amount in HB, as in the case of the origin of the induction period shown in Figure 2b (see the details in the Supporting Information). We can hence at least classify that the HB and heated HB (hydrogen-deficient-HB) have different catalytic properties (i.e., different acid sites). However, the active sites cannot be solely determined by our current experimental results. At least it should be clarified whether the hydrogen-deficient HB consists of a uniform stoichiometric structure or defective structure. Further investigation is thus required to determine the exact active sites of the HB solid- acid catalyst, e.g., the careful structure characterization, infrared spectroscopy analysis with pyridine, NH3, and CO2 adsorption, and H0 measurements with quantitative density, as a function of the pretreatment heating temperature (i.e., hydrogen amount in HB). Figure 4. Arrhenius plot of the ethanol-reforming reaction rate (k) in the presence of HB sheets for W/F = 27.2, 13.6, and 6.7 g·min/mmol. reaction, as k = (C2H5OH conversion [%])/100 × (C2H5OH flux [mol/s])/(HB amount [mol]), for the results under various W/F conditions with the HB sheets. As the obtained linear lines are almost the same and they are independent of W/F, the reaction can be (at least apparently) considered a first-order reaction. To determine the exact reaction order and mechanism, further kinetic analysis is required. From the slope and the section, the apparent activation energy Ea and pre- exponential factor A were estimated to be 102.8 ± 5.5 kJ/mol and 3.5 × 104 s−1, respectively. The derived Ea is comparable to the reported activation energies for the catalytic dehydration of ethanol over Al2O3 (53−155 kJ/mol),20,21 the Lewis acidic Zr- KIT-6 catalyst (79 kJ/mol),22 silica−alumina (125.5 kJ/ mol),23 and microporous Fe-ZSM-5 (137.7−271.1 kJ/mol).24 The formation rate of ethylene on our HB catalyst at 573 K and W/F = 7.3 g·min/mmol was 2.4 ± 0.1 mmol/g·h (Figure 2). Although this is not the formation rate at the optimal catalytic condition, here, we compare it with those of the other reported catalysts. Chen and co-workers reported that the formation rate of ethylene from ethanol using the commercial SynDol (Al2O3−MgO/SiO2) catalyst was 7.8 mmol/g·h at 591 K, with a weight hourly space velocity (WHSV) of 0.23 h−1 in a fixed-bed reactor (this value is calculated using the reported yield of the ethylene, which is 0.22 g/gcat·h).25 They also reported a higher formation rate of 535 mmol/g·h on Ti/γ- Al2O3 at 633 K and WHSV of 26 h−1 using the microreactor (calculated using a value of 15 g/gcat·h).25 The formation rate of HB sheets is, thus, lower than that of the state-of-the-art catalysts but is in the same order as that of the commercial SynDol catalyst. Finally, we herein discuss the possible reaction mechanism and active sites as well as the acid property of the HB sheets. In the case of ethanol reforming on a zeolite catalyst,26−32 diethyl ether (C2H5OC2H5) is formed as a result of a two-molecule reaction. Diethyl ether then converts to ethylene at strong Brønsted acid sites. The number and the strength of strong Brønsted acid sites in the catalyst are thus reported to determine the catalytic activity for ethylene formation. Since HB sheets show weaker H0 (1.5 ≥ H0 ≥ 0.43)13 compared with that of zeolites (e.g., −3.0 ≥ H0 ≥ −8.2 of H-Zeolite),33 we can expect the formation of diethyl ether rather than ethylene on HB sheets, based on the trend of zeolite. However, we observed ethylene instead of diethyl ether on the HB sheets (Figures 2 and 3). Thus, the reaction is considered to proceed as a single molecule reaction via the formation of an ethoxy 14102 ■ CONCLUSIONS We found that hydrogen boride or hydrogenated borophene sheets catalyze the conversion of ethanol to ethylene and water above 493 K, with high selectivity, independent of the contact time, and an apparent activation energy of 102.8 ± 5.5 kJ/mol. We hence consider hydrogenated borophene sheets to be novel, nonmetal, and two-dimensional solid-acid catalysts that have great potential for application as hydrogen-storage materials and in batteries. ■ Materials. HB sheets were prepared using a previously reported ion-exchange method.13 Specifically, MgB2 powder (1.0 g, 99%, Rare Metallic Co., Ltd., Tokyo, Japan) in acetonitrile (300 mL, 99.5%, Wako Pure Chemical Industries DOI: 10.1021/acsomega.9b02020 ACS Omega 2019, 4, 14100−14104 EXPERIMENTAL SECTIONPDF Image | Hydrogenated Borophene Shows Catalytic Activity as Solid Acid
PDF Search Title:
Hydrogenated Borophene Shows Catalytic Activity as Solid AcidOriginal File Name Searched:
Hydrogenated_Borophene_Shows_Catalytic_Activity_as.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |