
PDF Publication Title:
Text from PDF Page: 005
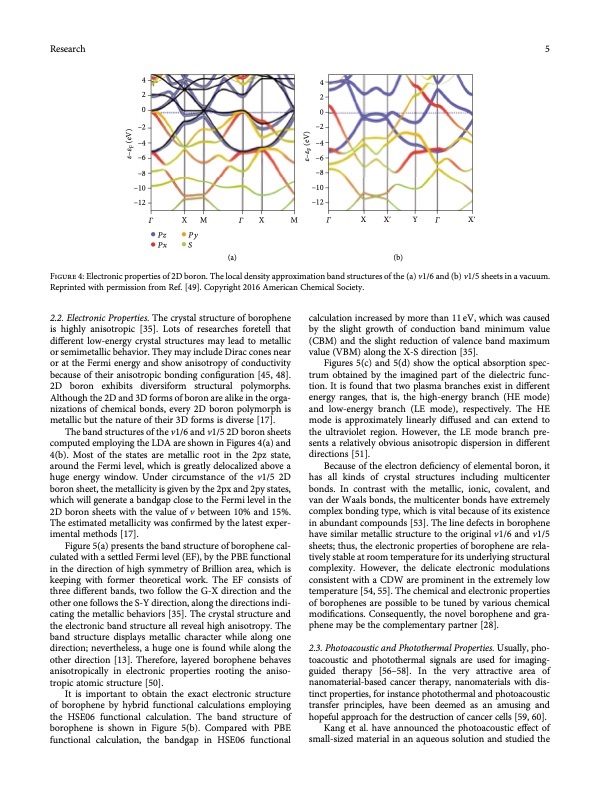
Research 5 4 2 0 –2 –4 –6 –8 –10 –12 4 2 0 –2 –4 –6 –8 –10 –12 𝛤 X M 𝛤 X M 𝛤 X Xʹ Y 𝛤 Xʹ Pz Py Px S (a) (b) Figure 4: Electronic properties of 2D boron. The local density approximation band structures of the (a) v1/6 and (b) v1/5 sheets in a vacuum. Reprinted with permission from Ref. [49]. Copyright 2016 American Chemical Society. 2.2. Electronic Properties. The crystal structure of borophene is highly anisotropic [35]. Lots of researches foretell that different low-energy crystal structures may lead to metallic or semimetallic behavior. They may include Dirac cones near or at the Fermi energy and show anisotropy of conductivity because of their anisotropic bonding configuration [45, 48]. 2D boron exhibits diversiform structural polymorphs. Although the 2D and 3D forms of boron are alike in the orga- nizations of chemical bonds, every 2D boron polymorph is metallic but the nature of their 3D forms is diverse [17]. The band structures of the v1/6 and v1/5 2D boron sheets computed employing the LDA are shown in Figures 4(a) and 4(b). Most of the states are metallic root in the 2pz state, around the Fermi level, which is greatly delocalized above a huge energy window. Under circumstance of the v1/5 2D boron sheet, the metallicity is given by the 2px and 2py states, which will generate a bandgap close to the Fermi level in the 2D boron sheets with the value of v between 10% and 15%. The estimated metallicity was confirmed by the latest exper- imental methods [17]. Figure 5(a) presents the band structure of borophene cal- culated with a settled Fermi level (EF), by the PBE functional in the direction of high symmetry of Brillion area, which is keeping with former theoretical work. The EF consists of three different bands, two follow the G-X direction and the other one follows the S-Y direction, along the directions indi- cating the metallic behaviors [35]. The crystal structure and the electronic band structure all reveal high anisotropy. The band structure displays metallic character while along one direction; nevertheless, a huge one is found while along the other direction [13]. Therefore, layered borophene behaves anisotropically in electronic properties rooting the aniso- tropic atomic structure [50]. It is important to obtain the exact electronic structure of borophene by hybrid functional calculations employing the HSE06 functional calculation. The band structure of borophene is shown in Figure 5(b). Compared with PBE functional calculation, the bandgap in HSE06 functional calculation increased by more than 11 eV, which was caused by the slight growth of conduction band minimum value (CBM) and the slight reduction of valence band maximum value (VBM) along the X-S direction [35]. Figures 5(c) and 5(d) show the optical absorption spec- trum obtained by the imagined part of the dielectric func- tion. It is found that two plasma branches exist in different energy ranges, that is, the high-energy branch (HE mode) and low-energy branch (LE mode), respectively. The HE mode is approximately linearly diffused and can extend to the ultraviolet region. However, the LE mode branch pre- sents a relatively obvious anisotropic dispersion in different directions [51]. Because of the electron deficiency of elemental boron, it has all kinds of crystal structures including multicenter bonds. In contrast with the metallic, ionic, covalent, and van der Waals bonds, the multicenter bonds have extremely complex bonding type, which is vital because of its existence in abundant compounds [53]. The line defects in borophene have similar metallic structure to the original v1/6 and v1/5 sheets; thus, the electronic properties of borophene are rela- tively stable at room temperature for its underlying structural complexity. However, the delicate electronic modulations consistent with a CDW are prominent in the extremely low temperature [54, 55]. The chemical and electronic properties of borophenes are possible to be tuned by various chemical modifications. Consequently, the novel borophene and gra- phene may be the complementary partner [28]. 2.3. Photoacoustic and Photothermal Properties. Usually, pho- toacoustic and photothermal signals are used for imaging- guided therapy [56–58]. In the very attractive area of nanomaterial-based cancer therapy, nanomaterials with dis- tinct properties, for instance photothermal and photoacoustic transfer principles, have been deemed as an amusing and hopeful approach for the destruction of cancer cells [59, 60]. Kang et al. have announced the photoacoustic effect of small-sized material in an aqueous solution and studied the 𝜀–𝜀F (eV) 𝜀–𝜀F (eV)PDF Image | Two-Dimensional Borophene
PDF Search Title:
Two-Dimensional BoropheneOriginal File Name Searched:
borophene.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |