
PDF Publication Title:
Text from PDF Page: 006
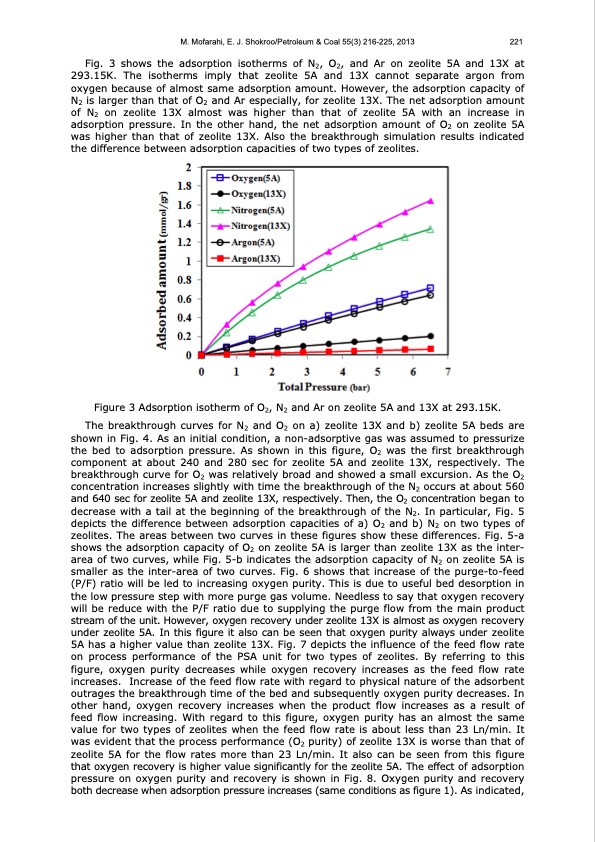
M. Mofarahi, E. J. Shokroo/Petroleum & Coal 55(3) 216-225, 2013 221 Fig. 3 shows the adsorption isotherms of N2, O2, and Ar on zeolite 5A and 13X at 293.15K. The isotherms imply that zeolite 5A and 13X cannot separate argon from oxygen because of almost same adsorption amount. However, the adsorption capacity of N2 is larger than that of O2 and Ar especially, for zeolite 13X. The net adsorption amount of N2 on zeolite 13X almost was higher than that of zeolite 5A with an increase in adsorption pressure. In the other hand, the net adsorption amount of O2 on zeolite 5A was higher than that of zeolite 13X. Also the breakthrough simulation results indicated the difference between adsorption capacities of two types of zeolites. Figure 3 Adsorption isotherm of O2, N2 and Ar on zeolite 5A and 13X at 293.15K. The breakthrough curves for N2 and O2 on a) zeolite 13X and b) zeolite 5A beds are shown in Fig. 4. As an initial condition, a non-adsorptive gas was assumed to pressurize the bed to adsorption pressure. As shown in this figure, O2 was the first breakthrough component at about 240 and 280 sec for zeolite 5A and zeolite 13X, respectively. The breakthrough curve for O2 was relatively broad and showed a small excursion. As the O2 concentration increases slightly with time the breakthrough of the N2 occurs at about 560 and 640 sec for zeolite 5A and zeolite 13X, respectively. Then, the O2 concentration began to decrease with a tail at the beginning of the breakthrough of the N2. In particular, Fig. 5 depicts the difference between adsorption capacities of a) O2 and b) N2 on two types of zeolites. The areas between two curves in these figures show these differences. Fig. 5-a shows the adsorption capacity of O2 on zeolite 5A is larger than zeolite 13X as the inter- area of two curves, while Fig. 5-b indicates the adsorption capacity of N2 on zeolite 5A is smaller as the inter-area of two curves. Fig. 6 shows that increase of the purge-to-feed (P/F) ratio will be led to increasing oxygen purity. This is due to useful bed desorption in the low pressure step with more purge gas volume. Needless to say that oxygen recovery will be reduce with the P/F ratio due to supplying the purge flow from the main product stream of the unit. However, oxygen recovery under zeolite 13X is almost as oxygen recovery under zeolite 5A. In this figure it also can be seen that oxygen purity always under zeolite 5A has a higher value than zeolite 13X. Fig. 7 depicts the influence of the feed flow rate on process performance of the PSA unit for two types of zeolites. By referring to this figure, oxygen purity decreases while oxygen recovery increases as the feed flow rate increases. Increase of the feed flow rate with regard to physical nature of the adsorbent outrages the breakthrough time of the bed and subsequently oxygen purity decreases. In other hand, oxygen recovery increases when the product flow increases as a result of feed flow increasing. With regard to this figure, oxygen purity has an almost the same value for two types of zeolites when the feed flow rate is about less than 23 Ln/min. It was evident that the process performance (O2 purity) of zeolite 13X is worse than that of zeolite 5A for the flow rates more than 23 Ln/min. It also can be seen from this figure that oxygen recovery is higher value significantly for the zeolite 5A. The effect of adsorption pressure on oxygen purity and recovery is shown in Fig. 8. Oxygen purity and recovery both decrease when adsorption pressure increases (same conditions as figure 1). As indicated,PDF Image | PRESSURE SWING ADSORPTION PROCESSES FOR AIR SEPARATION WITH ZEOLITE
PDF Search Title:
PRESSURE SWING ADSORPTION PROCESSES FOR AIR SEPARATION WITH ZEOLITEOriginal File Name Searched:
pc_3_2013_javadi_221.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |