
PDF Publication Title:
Text from PDF Page: 005
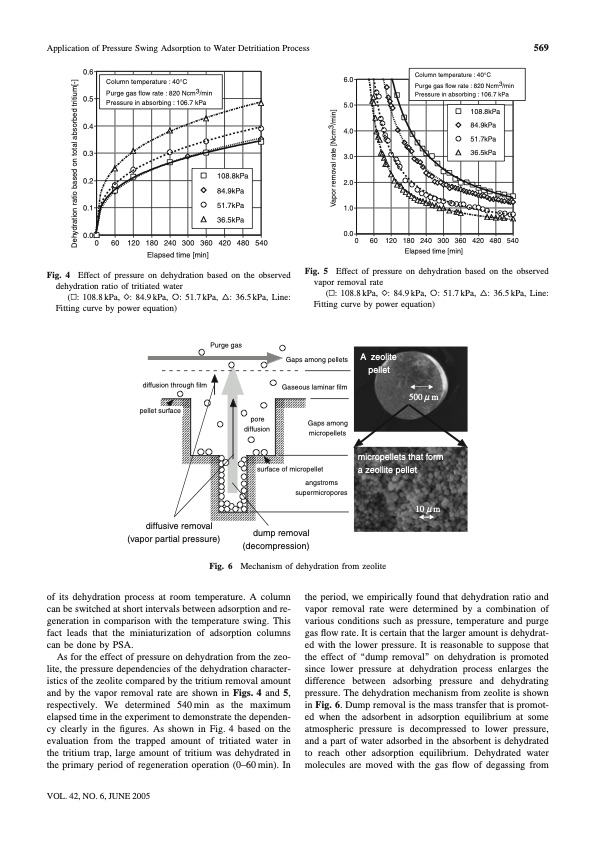
Application of Pressure Swing Adsorption to Water Detritiation Process 569 0.6 0.5 0.4 0.3 0.2 0.1 0.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 Column temperature : 40°C Purge gas flow rate : 820 Ncm3/min Pressure in absorbing : 106.7 kPa 108.8kPa 84.9kPa 51.7kPa 36.5kPa Column temperature : 40°C Purge gas flow rate : 820 Ncm3/min Pressure in absorbing : 106.7 kPa 108.8kPa 84.9kPa 51.7kPa 36.5kPa 0 60 120 180 240 300 360 420 480 540 Elapsed time [min] 0 60 120 180 240 300 360 420 480 540 Elapsed time [min] Fig. 4 Effect of pressure on dehydration based on the observed dehydration ratio of tritiated water Fig. 5 Effect of pressure on dehydration based on the observed vapor removal rate ( : 108.8 kPa, : 84.9 kPa, Fitting curve by power equation) : 51.7 kPa, : 36.5 kPa, Line: ( : 108.8 kPa, : 84.9 kPa, Fitting curve by power equation) : 51.7 kPa, : 36.5 kPa, Line: Purge gas diffusion through film pellet surface diffusive removal (vapor partial pressure) Fig. 6 Gaps among pellets Gaseous laminar film Gaps among micropellets A zeolite pellet micropellets that form a zeollite pellet 500 μ m pore diffusion of its dehydration process at room temperature. A column can be switched at short intervals between adsorption and re- generation in comparison with the temperature swing. This fact leads that the miniaturization of adsorption columns can be done by PSA. As for the effect of pressure on dehydration from the zeo- lite, the pressure dependencies of the dehydration character- istics of the zeolite compared by the tritium removal amount and by the vapor removal rate are shown in Figs. 4 and 5, respectively. We determined 540min as the maximum elapsed time in the experiment to demonstrate the dependen- cy clearly in the figures. As shown in Fig. 4 based on the evaluation from the trapped amount of tritiated water in the tritium trap, large amount of tritium was dehydrated in the primary period of regeneration operation (0–60 min). In the period, we empirically found that dehydration ratio and vapor removal rate were determined by a combination of various conditions such as pressure, temperature and purge gas flow rate. It is certain that the larger amount is dehydrat- ed with the lower pressure. It is reasonable to suppose that the effect of ‘‘dump removal’’ on dehydration is promoted since lower pressure at dehydration process enlarges the difference between adsorbing pressure and dehydrating pressure. The dehydration mechanism from zeolite is shown in Fig. 6. Dump removal is the mass transfer that is promot- ed when the adsorbent in adsorption equilibrium at some atmospheric pressure is decompressed to lower pressure, and a part of water adsorbed in the absorbent is dehydrated to reach other adsorption equilibrium. Dehydrated water molecules are moved with the gas flow of degassing from VOL. 42, NO. 6, JUNE 2005 surface of micropellet angstroms supermicropores dump removal (decompression) Mechanism of dehydration from zeolite 10 μ m Dehydration ratio based on total absorbed tritium[-] Vapor removal rate [Ncm3/min]PDF Image | Pressure Swing Adsorption to Water Detritiation Process
PDF Search Title:
Pressure Swing Adsorption to Water Detritiation ProcessOriginal File Name Searched:
PSA-Water-Detritiation-Process.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |