
PDF Publication Title:
Text from PDF Page: 006
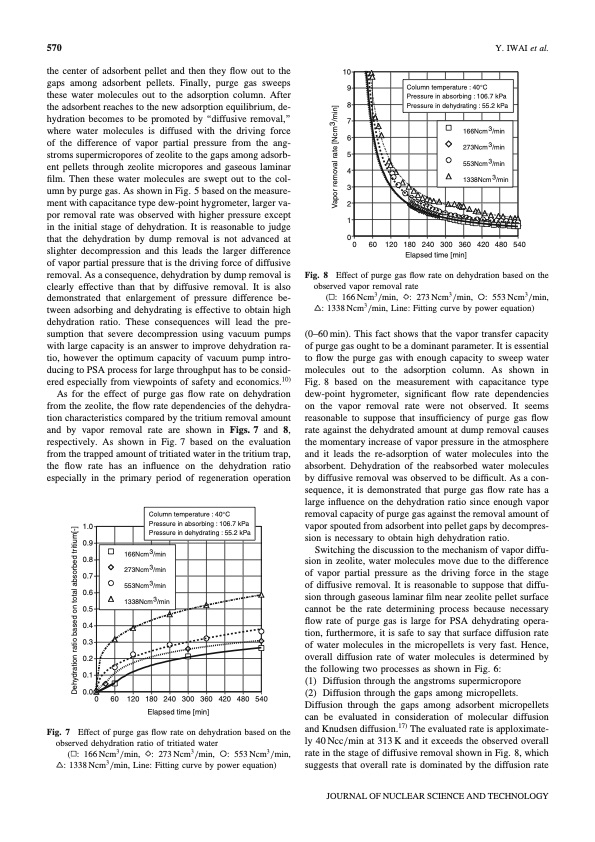
570 Y. IWAI et al. the center of adsorbent pellet and then they flow out to the gaps among adsorbent pellets. Finally, purge gas sweeps these water molecules out to the adsorption column. After the adsorbent reaches to the new adsorption equilibrium, de- hydration becomes to be promoted by ‘‘diffusive removal,’’ where water molecules is diffused with the driving force of the difference of vapor partial pressure from the ang- stroms supermicropores of zeolite to the gaps among adsorb- ent pellets through zeolite micropores and gaseous laminar film. Then these water molecules are swept out to the col- umn by purge gas. As shown in Fig. 5 based on the measure- ment with capacitance type dew-point hygrometer, larger va- por removal rate was observed with higher pressure except in the initial stage of dehydration. It is reasonable to judge that the dehydration by dump removal is not advanced at slighter decompression and this leads the larger difference of vapor partial pressure that is the driving force of diffusive removal. As a consequence, dehydration by dump removal is clearly effective than that by diffusive removal. It is also demonstrated that enlargement of pressure difference be- tween adsorbing and dehydrating is effective to obtain high dehydration ratio. These consequences will lead the pre- sumption that severe decompression using vacuum pumps with large capacity is an answer to improve dehydration ra- tio, however the optimum capacity of vacuum pump intro- ducing to PSA process for large throughput has to be consid- ered especially from viewpoints of safety and economics.10) As for the effect of purge gas flow rate on dehydration from the zeolite, the flow rate dependencies of the dehydra- tion characteristics compared by the tritium removal amount and by vapor removal rate are shown in Figs. 7 and 8, respectively. As shown in Fig. 7 based on the evaluation from the trapped amount of tritiated water in the tritium trap, the flow rate has an influence on the dehydration ratio especially in the primary period of regeneration operation 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 60 120 180 240 300 360 420 480 540 Elapsed time [min] Effect of purge gas flow rate on dehydration based on the observed dehydration ratio of tritiated water ( : 166 Ncm3 /min, : 273 Ncm3 /min, : 553 Ncm3 /min, : 1338Ncm3/min, Line: Fitting curve by power equation) 10 9 C olumn temperature : 40°C Pressure in absorbing : 106.7 kPa ressure in dehydrating : 55.2 kPa 166Ncm 3/min 273Ncm 3/min 553Ncm 3/min 1338Ncm 3/min Fig. 8 8P 7 6 5 4 3 2 1 0 0 60 120 180 240 300 360 420 480 540 Elapsed time [min] Effect of purge gas flow rate on dehydration based on the observed vapor removal rate ( : 166 Ncm3 /min, : 273 Ncm3 /min, : 553 Ncm3 /min, : 1338Ncm3/min, Line: Fitting curve by power equation) (0–60 min). This fact shows that the vapor transfer capacity of purge gas ought to be a dominant parameter. It is essential to flow the purge gas with enough capacity to sweep water molecules out to the adsorption column. As shown in Fig. 8 based on the measurement with capacitance type dew-point hygrometer, significant flow rate dependencies on the vapor removal rate were not observed. It seems reasonable to suppose that insufficiency of purge gas flow rate against the dehydrated amount at dump removal causes the momentary increase of vapor pressure in the atmosphere and it leads the re-adsorption of water molecules into the absorbent. Dehydration of the reabsorbed water molecules by diffusive removal was observed to be difficult. As a con- sequence, it is demonstrated that purge gas flow rate has a large influence on the dehydration ratio since enough vapor removal capacity of purge gas against the removal amount of vapor spouted from adsorbent into pellet gaps by decompres- sion is necessary to obtain high dehydration ratio. Switching the discussion to the mechanism of vapor diffu- sion in zeolite, water molecules move due to the difference of vapor partial pressure as the driving force in the stage of diffusive removal. It is reasonable to suppose that diffu- sion through gaseous laminar film near zeolite pellet surface cannot be the rate determining process because necessary flow rate of purge gas is large for PSA dehydrating opera- tion, furthermore, it is safe to say that surface diffusion rate of water molecules in the micropellets is very fast. Hence, overall diffusion rate of water molecules is determined by the following two processes as shown in Fig. 6: (1) Diffusion through the angstroms supermicropore (2) Diffusion through the gaps among micropellets. Diffusion through the gaps among adsorbent micropellets can be evaluated in consideration of molecular diffusion and Knudsen diffusion.17) The evaluated rate is apploximate- ly 40 Ncc/min at 313 K and it exceeds the observed overall rate in the stage of diffusive removal shown in Fig. 8, which suggests that overall rate is dominated by the diffusion rate 166Ncm 3/min 273Ncm 3/min 553Ncm 3/min 1338Ncm 3/min Column temperature : 40°C Pressure in absorbing : 106.7 kPa Pressure in dehydrating : 55.2 kPa Fig. 7 JOURNAL OF NUCLEAR SCIENCE AND TECHNOLOGY Dehydration ratio based on total absorbed tritium[-] Vapor removal rate [Ncm3/min]PDF Image | Pressure Swing Adsorption to Water Detritiation Process
PDF Search Title:
Pressure Swing Adsorption to Water Detritiation ProcessOriginal File Name Searched:
PSA-Water-Detritiation-Process.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |