
PDF Publication Title:
Text from PDF Page: 122
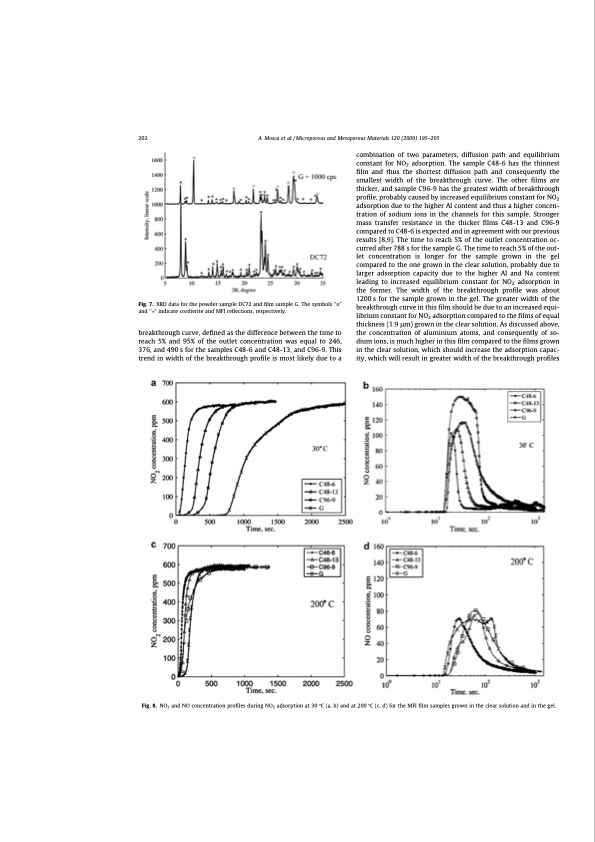
202 A. Mosca et al. / Microporous and Mesoporous Materials 120 (2009) 195–205 Fig. 7. XRD data for the powder sample DC72 and film sample G. The symbols ‘‘o” and ‘‘” indicate cordierite and MFI reflections, respectively. breakthrough curve, defined as the difference between the time to reach 5% and 95% of the outlet concentration was equal to 246, 376, and 490 s for the samples C48-6 and C48-13, and C96-9. This trend in width of the breakthrough profile is most likely due to a combination of two parameters, diffusion path and equilibrium constant for NO2 adsorption. The sample C48-6 has the thinnest film and thus the shortest diffusion path and consequently the smallest width of the breakthrough curve. The other films are thicker, and sample C96-9 has the greatest width of breakthrough profile, probably caused by increased equilibrium constant for NO2 adsorption due to the higher Al content and thus a higher concen- tration of sodium ions in the channels for this sample. Stronger mass transfer resistance in the thicker films C48-13 and C96-9 compared to C48-6 is expected and in agreement with our previous results [8,9]. The time to reach 5% of the outlet concentration oc- curred after 788 s for the sample G. The time to reach 5% of the out- let concentration is longer for the sample grown in the gel compared to the one grown in the clear solution, probably due to larger adsorption capacity due to the higher Al and Na content leading to increased equilibrium constant for NO2 adsorption in the former. The width of the breakthrough profile was about 1200 s for the sample grown in the gel. The greater width of the breakthrough curve in this film should be due to an increased equi- librium constant for NO2 adsorption compared to the films of equal thickness (1.9 lm) grown in the clear solution. As discussed above, the concentration of aluminium atoms, and consequently of so- dium ions, is much higher in this film compared to the films grown in the clear solution, which should increase the adsorption capac- ity, which will result in greater width of the breakthrough profiles Fig. 8. NO2 and NO concentration profiles during NO2 adsorption at 30 °C (a, b) and at 200 °C (c, d) for the MFI film samples grown in the clear solution and in the gel.PDF Image | Structured Zeolite Adsorbents for PSA Applications
PDF Search Title:
Structured Zeolite Adsorbents for PSA ApplicationsOriginal File Name Searched:
structured-zeolites.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |