
PDF Publication Title:
Text from PDF Page: 007
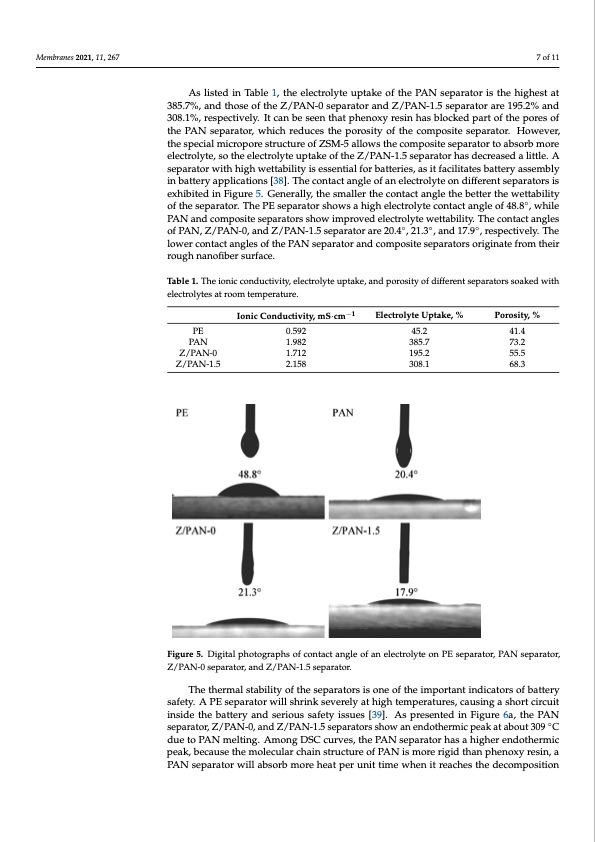
Membranes 2021, 11, 267 7 of 11 Membranes 2021, 11, x FOR PEER REVIEW 7 of 11 As listed in Table 1, the electrolyte uptake of the PAN separator is the highest at 385.7%, and those of the Z/PAN-0 separator and Z/PAN-1.5 separator are 195.2% and As listed in Table 1, the electrolyte uptake of the PAN separator is the highest at 308.1%, respectively. It can be seen that phenoxy resin has blocked part of the pores of 385.7%, and those of the Z/PAN-0 separator and Z/PAN-1.5 separator are 195.2% and the PAN separator, which reduces the porosity of the composite separator. However, 308.1%, respectively. It can be seen that phenoxy resin has blocked part of the pores of the the special micropore structure of ZSM-5 allows the composite separator to absorb more PAN separator, which reduces the porosity of the composite separator. However, the spe- electrolyte, so the electrolyte uptake of the Z/PAN-1.5 separator has decreased a little. A cial micropore structure of ZSM-5 allows the composite separator to absorb more electro- separator with high wettability is essential for batteries, as it facilitates battery assembly lyte, so the electrolyte uptake of the Z/PAN-1.5 separator has decreased a little. A separa- in battery applications [38]. The contact angle of an electrolyte on different separators is tor with high wettability is essential for batteries, as it facilitates battery assembly in bat- exhibited in Figure 5. Generally, the smaller the contact angle the better the wettability tery applications [38]. The contact angle of an electrolyte on different separators◦is exhib- of the separator. The PE separator shows a high electrolyte contact angle of 48.8 , while ited in Figure 5. Generally, the smaller the contact angle the better the wettability of the PAN and composite separators show improved electrolyte wettability. The contact angles separator. The PE separator shows a high electrolyte contact angle of 48.8°, while PAN of PAN, Z/PAN-0, and Z/PAN-1.5 separator are 20.4◦, 21.3◦, and 17.9◦, respectively. The and composite separators show improved electrolyte wettability. The contact angles of lower contact angles of the PAN separator and composite separators originate from their PAN, Z/PAN-0, and Z/PAN-1.5 separator are 20.4°, 21.3°, and 17.9°, respectively. The rough nanofiber surface. lower contact angles of the PAN separator and composite separators originate from their rough nanofiber surface. Table 1. The ionic conductivity, electrolyte uptake, and porosity of different separators soaked with electrolytes at room temperature. Table 1. The ionic conductivity, electrolyte uptake, and porosity of different separators soaked PE PAN Z/PAN-0 Z/PAN-1.5 PAN Z/PAN-0 Z/PAN-1.5 0.592 1.982 1.712 2.158 1.982 1.712 2.158 45.2 385.7 195.2 308.1 41.4 with electrolytes at room temperature. −1 Ionic Conductivity, mS·cm Electrolyte Uptake, % Porosity, % Poro4s1i.t4y, % 73.2 −1 IoniPcEConductivity, mS·c0m.592 Electrolyte Upta4k5e.2, % 385.7 195.2 308.1 55.5 73.2 68.3 55.5 68.3 Figure 5. Digital photographs of contact angle of an electrolyte on PE separator, PAN separator, Figure 5. Digital photographs of contact angle of an electrolyte on PE separator, PAN separator, Z/PAN-0 separator, and Z/PAN-1.5 separator. Z/PAN-0 separator, and Z/PAN-1.5 separator. The thermal stability of the separators is one of the important indicators of battery The thermal stability of the separators is one of the important indicators of battery safety. A PE separator will shrink severely at high temperatures, causing a short circuit safety. A PE separator will shrink severely at high temperatures, causing a short circuit inside the battery and serious safety issues [39]. As presented in Figure 6a, the PAN sep- inside the battery and serious safety issues [39]. As presented in Figure 6a, the PAN arator, Z/PAN-0, and Z/PAN-1.5 separators show an endothermic peak at about 309◦ °C separator, Z/PAN-0, and Z/PAN-1.5 separators show an endothermic peak at about 309 C due to PAN melting. Among DSC curves, the PAN separator has a higher endothermic due to PAN melting. Among DSC curves, the PAN separator has a higher endothermic peak, because the molecular chain structure of PAN is more rigid than phenoxy resin, a peak, because the molecular chain structure of PAN is more rigid than phenoxy resin, a PAN separator will absorb more heat per unit time when it reaches the decomposition PAN separator will absorb more heat per unit time when it reaches the decomposition temperature than the Z/PAN-0 separator. The melting temperature of PE separators isPDF Image | Electrospinning Polyacrylonitrile Separator with Dip-Coating of Zeolite
PDF Search Title:
Electrospinning Polyacrylonitrile Separator with Dip-Coating of ZeoliteOriginal File Name Searched:
membranes-11-00267.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |