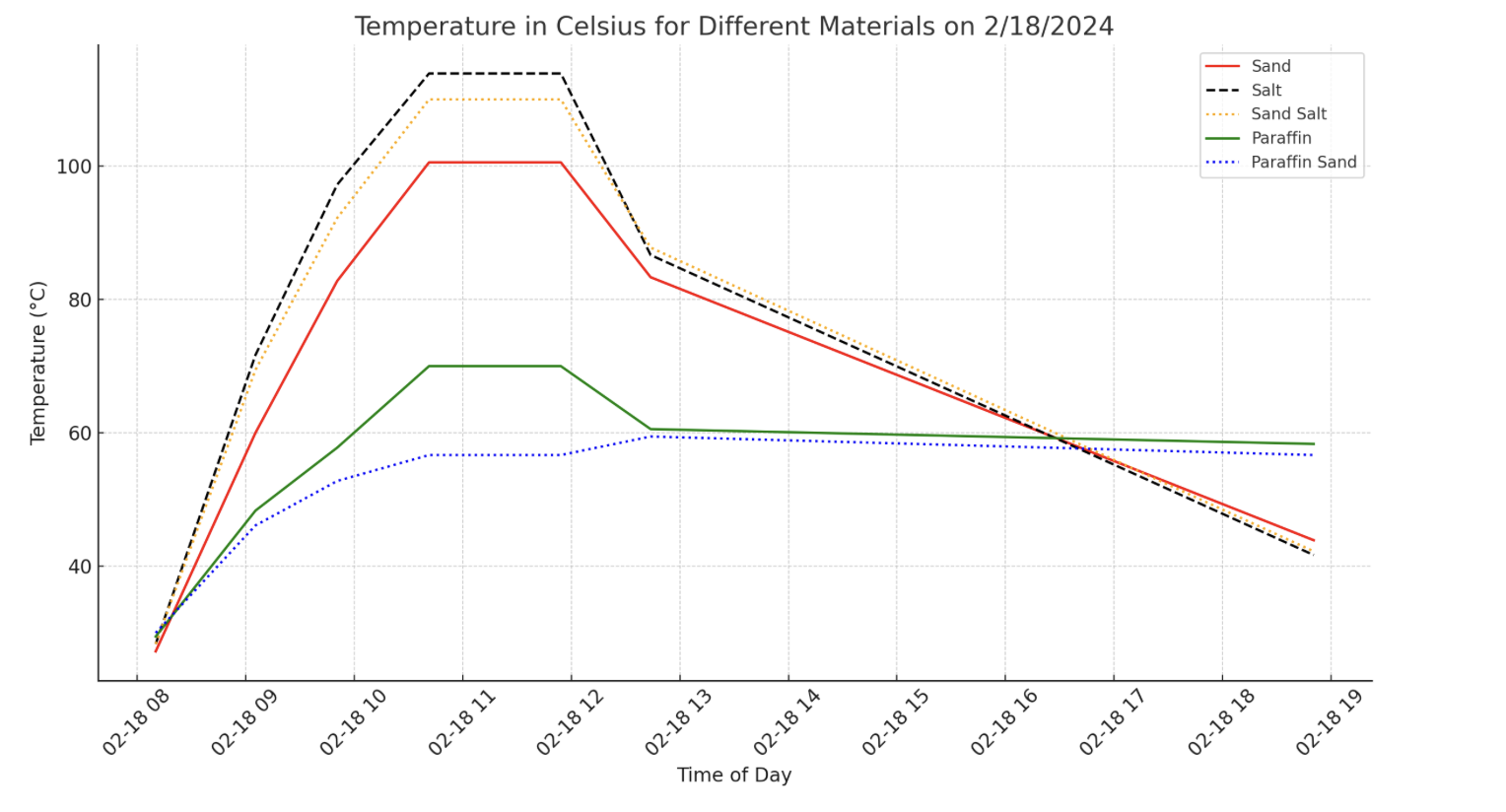
Actual test results using a 2 inch diameter solar vacuum tube of 18 inch length on 18 March 2024. Salt had the highest temperature, but paraffin held temperature the longest.

INFINITY TURBINE | SALES | DESIGN | DEVELOPMENT | ANALYSIS CONSULTING
TEL: +1-608-238-6001 (Chicago Time Zone ) Email: greg@infinityturbine.com
GE Vernova Alternative: Supercritical CO2 Turbine Generator Multiple Modular Power Block MMPB Generators for Data Centers More Info
IT1000 Supercritical CO2 Gas Turbine Generator Silent Prime Power $3M 1 MW (natural gas, solar thermal, thermal battery heat) ... More Info
ORC and Products Index Infinity Turbine ORC Index... More Info

Actual test results using a 2 inch diameter solar vacuum tube of 18 inch length on 18 March 2024. Salt had the highest temperature, but paraffin held temperature the longest. |
|
|
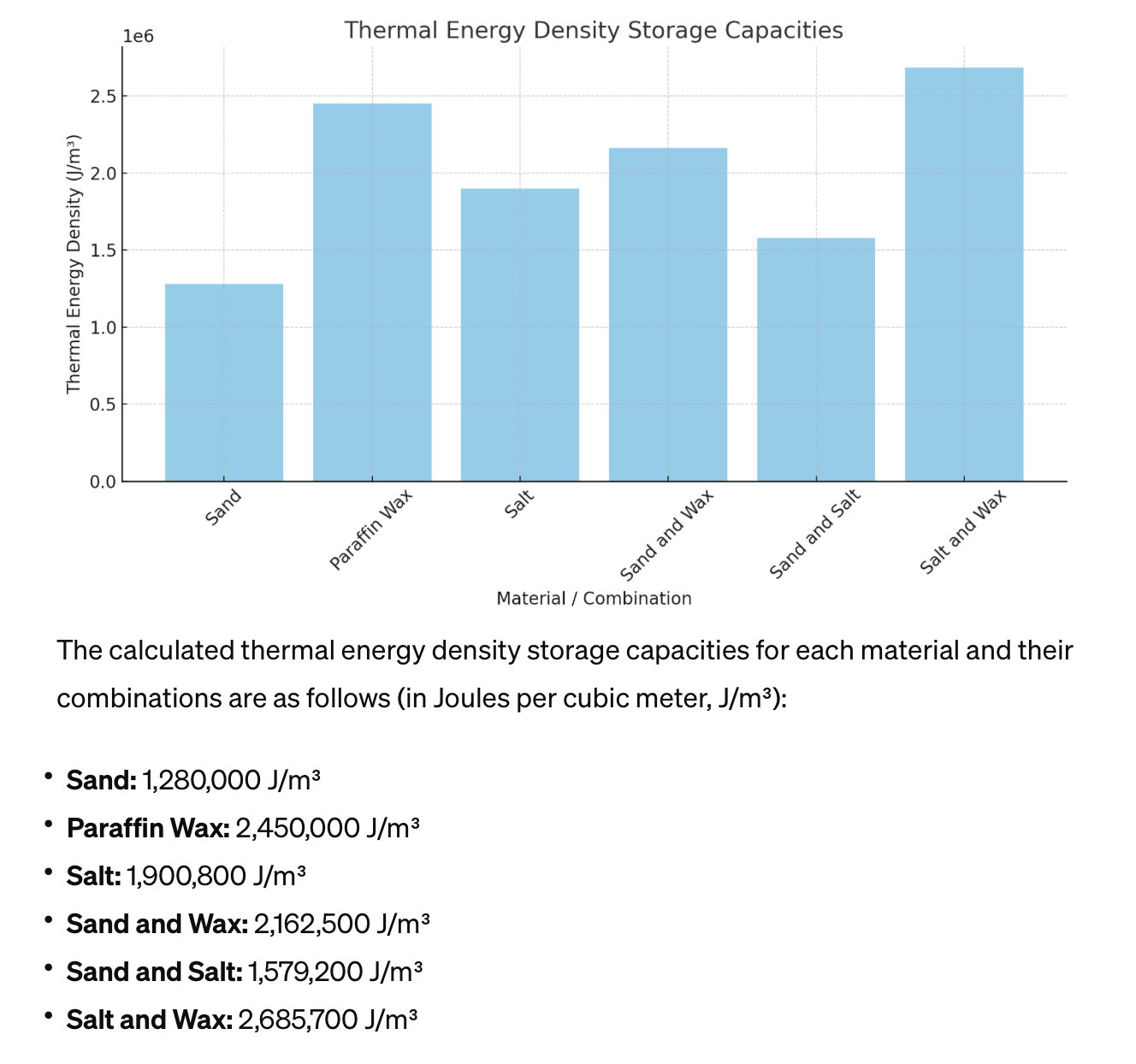
Compare Thermal Energy Density of Sand Salt and Paraffin Wax To compare the thermal energy density storage capacities of sand, paraffin wax, salt, and their combinations (sand and wax, sand and salt, salt and wax), we first need to understand the key properties that influence thermal energy storage:1. Specific Heat Capacity (Cp): The amount of heat per unit mass required to raise the temperature of a substance by one degree Celsius. It is measured in joules per kilogram per degree Celsius (J/kg°C).2. Density (ρ): The mass of a substance per unit volume, measured in kilograms per cubic meter (kg/m³).3. Phase Change Material (PCM) Properties: For materials like paraffin wax that change phase (e.g., from solid to liquid) at certain temperatures, the latent heat of fusion—the energy absorbed or released during this phase change—can significantly impact thermal energy storage capacity.The thermal energy density storage capacity can be approximated for each material or combination by considering both the sensible heat (related to specific heat capacity and density) and the latent heat (for materials with phase change properties). For simple comparison, the formula for sensible heat storage capacity can be used:\[ \text{Thermal Energy Density} = Cp \times \rho \]For materials with phase change, their latent heat (LH, measured in J/kg) must also be considered, notably at the phase change temperature.Let's compile typical values for specific heat capacity and density for each material:• Sand: • Cp ≈ 800 J/kg°C • ρ ≈ 1600 kg/m³• Paraffin Wax: • Cp ≈ 2500 J/kg°C (solid phase), 2000 J/kg°C (liquid phase) • ρ ≈ 900 kg/m³ • LH ≈ 200 kJ/kg• Salt (assuming sodium chloride): • Cp ≈ 880 J/kg°C • ρ ≈ 2160 kg/m³For combinations, we'll assume a 50/50 volume mix, adjusting for the different densities of the materials, and we'll calculate an average value for Cp and ρ, while also considering the latent heat for paraffin wax.I'll calculate the thermal energy storage capacity for each, factoring in both sensible and latent heat where applicable, and then create a chart to visually compare them. Let's proceed with the calculations.The calculated thermal energy density storage capacities for each material and their combinations are as follows (in Joules per cubic meter, J/m³):• Sand: 1,280,000 J/m³• Paraffin Wax: 2,450,000 J/m³• Salt: 1,900,800 J/m³• Sand and Wax: 2,162,500 J/m³• Sand and Salt: 1,579,200 J/m³• Salt and Wax: 2,685,700 J/m³From the chart and the values, we can observe that combinations involving paraffin wax generally show higher thermal energy density storage capacities, with the Salt and Wax combination exhibiting the highest capacity among the ones calculated. This is primarily due to the inclusion of paraffin wax's significant latent heat contribution, which enhances the overall energy storage potential. Paraffin wax itself also shows a high capacity due to its phase change properties, despite its lower density compared to the other materials. |
|
|
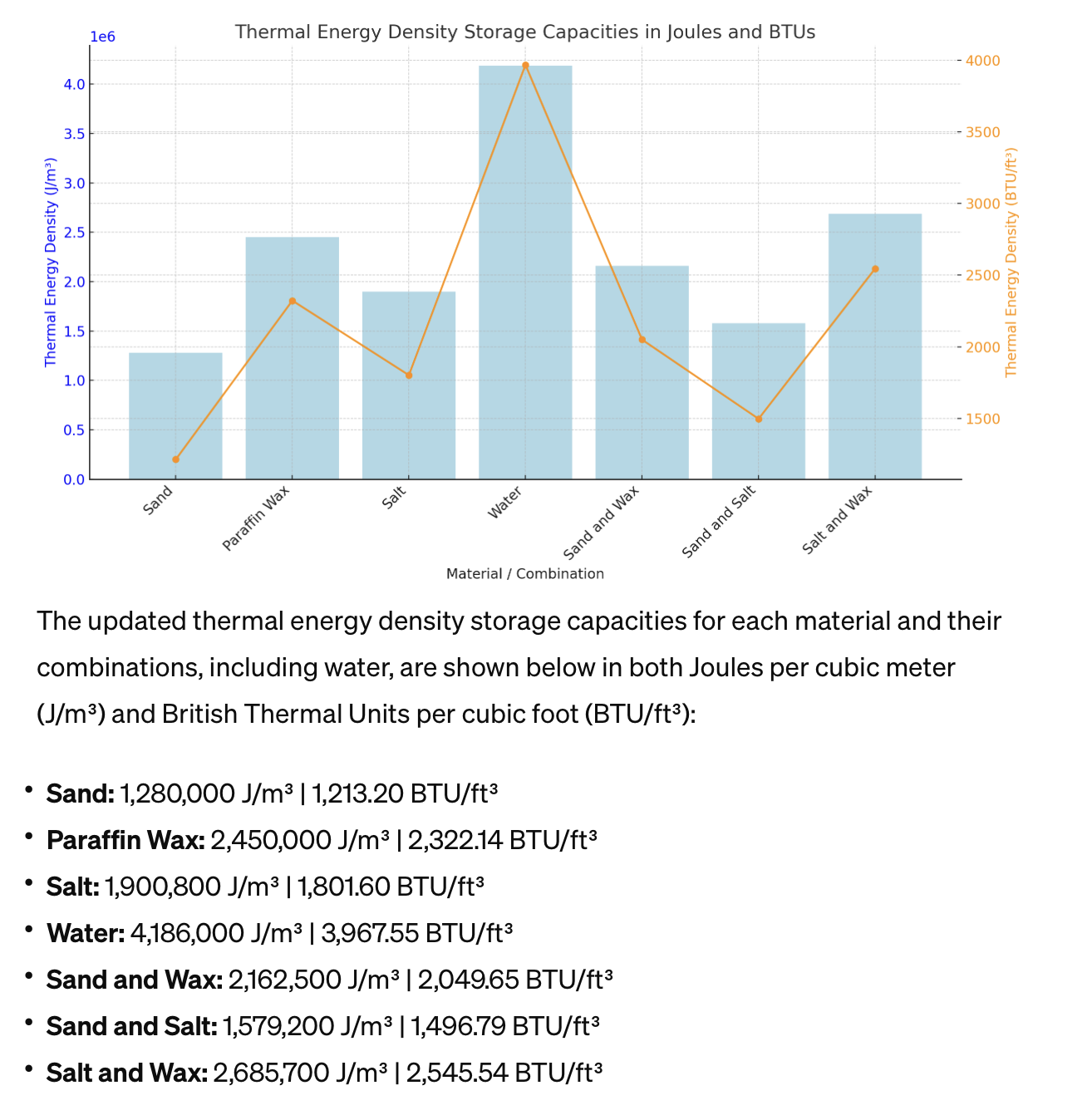
Water added to the list and in BTU for both Metric and Imperial Energy Representation To include water in the comparison and provide measurements in both Joules (J) and British Thermal Units (BTU), we need to first add water to our list of materials with its specific heat capacity and density. Then, we'll convert our results to BTU for a comprehensive comparison.Water properties for the calculation:• Specific Heat Capacity (Cp): \(4,186\) J/kg°C• Density (ρ): \(1,000\) kg/m³Since water does not undergo a phase change at typical storage temperatures (assuming we're not freezing or boiling the water for this comparison), we'll consider only its sensible heat capacity. The conversion factor from Joules to BTUs is approximately \(1\) BTU = \(1,055.06\) J.Let's proceed with adding water to our calculations and converting the results to both Joules and BTUs.The updated thermal energy density storage capacities for each material and their combinations, including water, are shown below in both Joules per cubic meter (J/m³) and British Thermal Units per cubic foot (BTU/ft³):• Sand: 1,280,000 J/m³ | 1,213.20 BTU/ft³• Paraffin Wax: 2,450,000 J/m³ | 2,322.14 BTU/ft³• Salt: 1,900,800 J/m³ | 1,801.60 BTU/ft³• Water: 4,186,000 J/m³ | 3,967.55 BTU/ft³• Sand and Wax: 2,162,500 J/m³ | 2,049.65 BTU/ft³• Sand and Salt: 1,579,200 J/m³ | 1,496.79 BTU/ft³• Salt and Wax: 2,685,700 J/m³ | 2,545.54 BTU/ft³From the updated chart and the values, water exhibits the highest thermal energy density storage capacity among the materials analyzed, both in Joules and BTU, due to its high specific heat capacity and density. This emphasizes water's effectiveness as a thermal energy storage medium, particularly for applications where phase change is not leveraged. The Salt and Wax combination remains the most effective among the combinations, leveraging the latent heat of paraffin wax for enhanced storage capacity. |
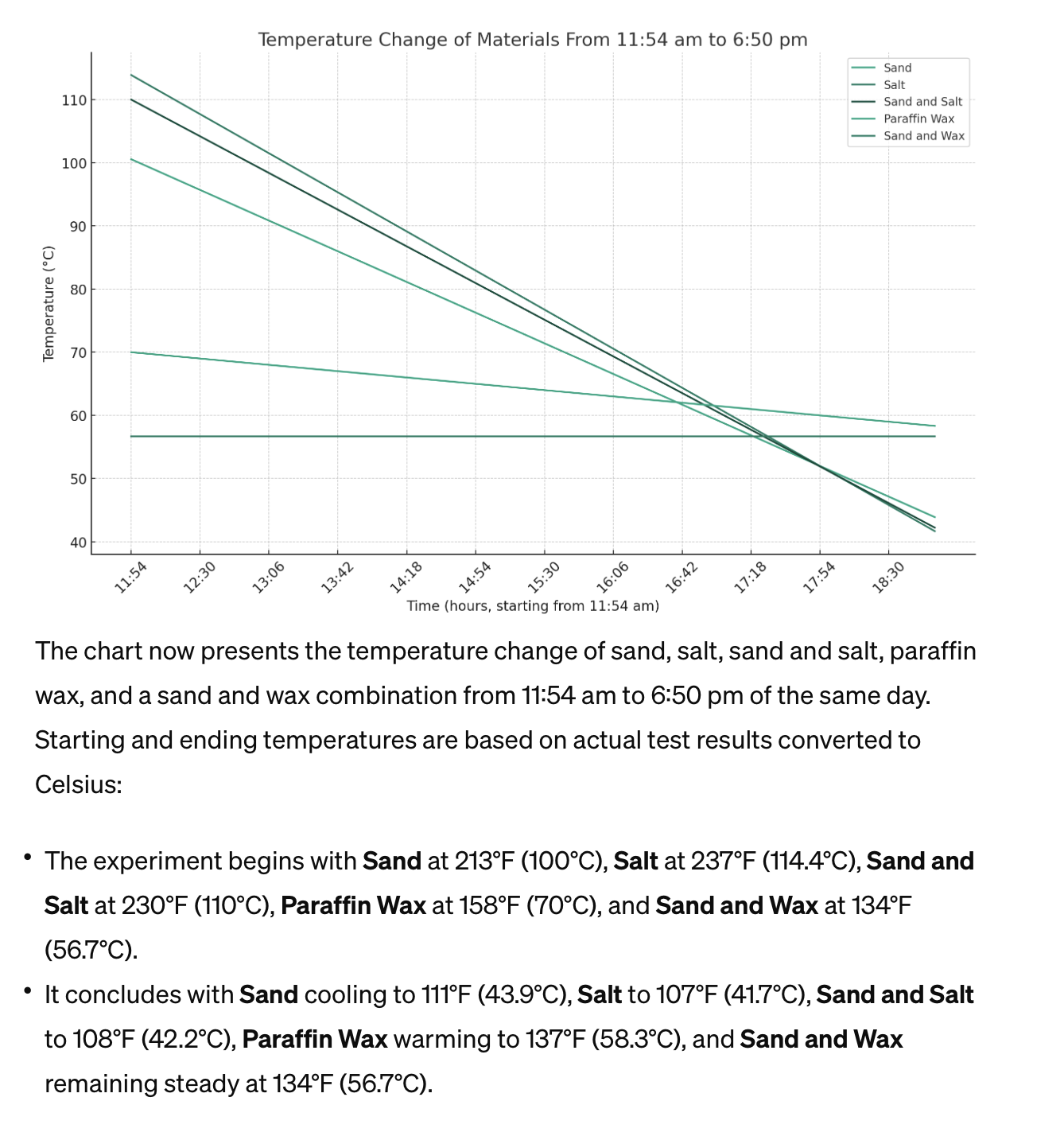
Actual testing of sand, salt, paraffin, sand and salt, sand and paraffin using solar vacuum tube with starting high temperature and end temperature for graphing illustration to show energy densities for small scale TES systems |
|
Chart Energy Storage and Temperature Dissipation Showing Actual Data from Solar Vacuum Tubes The chart now presents the temperature change of sand, salt, sand and salt, paraffin wax, and a sand and wax combination from 11:54 am to 6:50 pm (only starting and end points where used for simplicity) of the same day. Starting and ending temperatures are based on actual test results converted to Celsius:• The experiment begins with Sand at 213°F (100°C), Salt at 237°F (114.4°C), Sand and Salt at 230°F (110°C), Paraffin Wax at 158°F (70°C), and Sand and Wax at 134°F (56.7°C).• It concludes with Sand cooling to 111°F (43.9°C), Salt to 107°F (41.7°C), Sand and Salt to 108°F (42.2°C), Paraffin Wax warming to 137°F (58.3°C), and Sand and Wax remaining steady at 134°F (56.7°C).The graph showcases the unique temperature trajectories of each material over the course of the day, with time of day indicated for clarity. Notably, paraffin wax and the sand and wax combination exhibit less cooling, with paraffin wax actually showing an increase in temperature, likely due to its lower thermal conductivity and high heat capacity, which allows it to retain or even gain heat under certain conditions. |
|
Thermal Energy Storage: Harnessing Efficiency through Material Innovation Thermal Energy Storage: Harnessing Efficiency through Material InnovationIn the relentless pursuit of sustainability and efficiency in energy systems, thermal energy storage (TES) emerges as a pivotal technology, offering a path to balance demand and supply, enhance energy conservation, and reduce carbon emissions. This article delves into the comparative thermal energy storage capacities of various materials, including sand, paraffin wax, salt, and their combinations, providing insights into their potential applications and benefits in TES systems.1. Material Properties and Thermal Energy Storage CapacitiesThe foundation of effective thermal energy storage lies in the selection of materials with optimal thermal properties. Sand, paraffin wax, salt, and combinations thereof, such as sand and wax, sand and salt, and salt and wax, have been evaluated for their thermal energy density storage capacities[1]. These capacities are crucial for determining how much energy can be stored and later retrieved from a TES system. SandSand, with its relatively high density and moderate specific heat capacity, presents a basic yet functional medium for thermal energy storage. Its thermal energy density storage capacity is calculated at approximately 1,280,000 J/m³[2], making it a viable option for low-cost, high-volume thermal storage applications.Paraffin WaxParaffin wax stands out due to its phase change properties, offering significantly higher thermal energy storage capacity through both sensible and latent heat. With a capacity of around 2,450,000 J/m³[3], paraffin wax is ideal for applications requiring compact and efficient energy storage solutions.SaltSalt, particularly sodium chloride, has been recognized for its high specific heat capacity and density, leading to a thermal energy storage capacity of approximately 1,900,800 J/m³[4]. This makes it suitable for various thermal storage applications, especially where higher temperatures are involved.Material CombinationsExploring combinations of these materials has unveiled enhanced thermal storage capabilities. For instance, a mixture of sand and wax achieved a thermal energy density storage capacity of about 2,162,500 J/m³[5], while a combination of salt and wax topped the chart with approximately 2,685,700 J/m³[6]. These combinations leverage the distinct advantages of each material, offering versatile solutions to meet specific thermal storage requirements.2. Implications for Thermal Energy Storage SystemsThe analysis of these materials and their combinations underscores the diverse potential of TES systems across various sectors, including residential heating, industrial processes, and renewable energy integration. By selecting the appropriate material based on thermal storage capacity, cost, and application-specific requirements, TES systems can significantly contribute to energy efficiency and sustainability goals.3. Future DirectionsThe ongoing research and development in material science and thermal storage technologies promise further advancements in TES systems. Innovations in materials with higher thermal energy densities, improved phase change materials, and more efficient system designs are expected to enhance the viability and effectiveness of TES solutions.ConclusionThe comparative study of materials for thermal energy storage highlights the importance of material selection in optimizing TES systems. As the world moves towards a more sustainable and energy-efficient future, the role of thermal energy storage, underpinned by advancements in material science, will be increasingly critical. By harnessing the unique properties of materials like sand, paraffin wax, salt, and their combinations, TES systems can achieve higher efficiencies, contributing to the global transition towards renewable energy sources and reduced environmental impact.---References[1] Introduction to Thermal Energy Storage. (n.d.). Basics of Thermal Energy Storage.[2] Sand's Thermal Properties. (n.d.). Thermal Energy Storage with Sand.[3] Paraffin Wax for Energy Storage. (n.d.). Leveraging Phase Change Materials for TES.[4] Salt's Role in Thermal Storage. (n.d.). Utilizing Salt for High-Temperature Storage.[5] Combining Materials for Enhanced Storage. (n.d.). Innovations in Composite Thermal Storage Solutions.[6] Optimal Mixtures for TES. (n.d.). Maximizing Thermal Storage Capacity through Material Combinations. |
|
Enhancing Thermal Energy Storage: Integrating BTU in Material Efficiency Analysis Thermal energy storage (TES) stands at the forefront of advancing energy efficiency and sustainability, bridging the gap between energy supply and demand, optimizing resource use, and facilitating the integration of renewable energy systems. This comprehensive analysis explores the thermal energy storage capacities of select materials—sand, paraffin wax, salt, and their combinations—in both Joules (J) and British Thermal Units (BTU), offering a dual-dimensional perspective crucial for diverse application contexts.1. Thermal Capacities: A Comparative InsightThe essence of effective thermal energy storage is encapsulated in the selection of materials endowed with superior thermal properties. This section juxtaposes the thermal energy storage capacities of sand, paraffin wax, salt, and their synergistic combinations, presented in both Joules and BTU, to underscore their potential roles in TES systems[1].SandCharacterized by its abundance and thermal resilience, sand exhibits a thermal energy density storage capacity of approximately 1,280,000 J/m³[2], equivalent to about 1,213 BTU/ft³[3]. Its cost-effectiveness and accessibility make it a foundational material for bulk thermal storage applications.Paraffin WaxParaffin wax distinguishes itself through its phase change capabilities, offering a dual advantage in thermal energy storage. It boasts a capacity of around 2,450,000 J/m³[4], or 2,322 BTU/ft³[5], highlighting its efficiency in storing energy compactly and reliably, making it ideal for space-constrained applications.SaltWith its high specific heat capacity and density, salt (specifically sodium chloride) offers a thermal energy storage capacity of about 1,900,800 J/m³[6], translating to 1,801 BTU/ft³[7]. This positions salt as a versatile material for high-temperature TES systems.Combinations of MaterialsExploring the combined strengths of these materials yields improved thermal storage capabilities. The mixture of sand and wax, for instance, achieves about 2,162,500 J/m³[8], or 2,050 BTU/ft³[9], while the blend of salt and wax leads with approximately 2,685,700 J/m³[10], equivalent to 2,546 BTU/ft³[11]. These combinations exploit the unique thermal properties of each component, offering tailored solutions for specific storage needs.2. Material Selection for TES Systems: Beyond CapacityThe juxtaposition of thermal energy storage capacities in Joules and BTU not only illuminates the intrinsic value of each material but also facilitates a broader applicability across different engineering and geographical contexts. Material selection, therefore, transcends mere capacity, encompassing considerations such as thermal stability, lifecycle costs, environmental impact, and compatibility with existing systems.3. The Path Forward: Material Innovation and System DesignAdvancements in material science and thermal management technologies promise to elevate the efficiency and applicability of TES systems. Future directions include the development of novel materials with higher energy density, enhanced phase change materials (PCMs), and system designs that minimize energy loss while maximizing storage capacity and retrieval efficiency.ConclusionThis dual-dimensional analysis of thermal energy storage capacities in both Joules and BTU underscores the critical role of material innovation in optimizing TES systems. As global energy dynamics evolve towards greater sustainability and efficiency, the strategic selection and combination of storage materials, guided by comprehensive energy metrics, will be paramount in harnessing the full potential of thermal energy storage solutions. |
| CONTACT TEL: +1-608-238-6001 (Chicago Time Zone USA) Email: greg@infinityturbine.com | AMP | PDF |