

TEL: 1-608-238-6001 Email: greg@infinityturbine.com
Infinity Turbine PowerBlock 10 MW Supercritical CO2 turbine generator power supplying 10 MW of power for AI Data Centers and charging Tesla MegaBlock... More Info

|
|
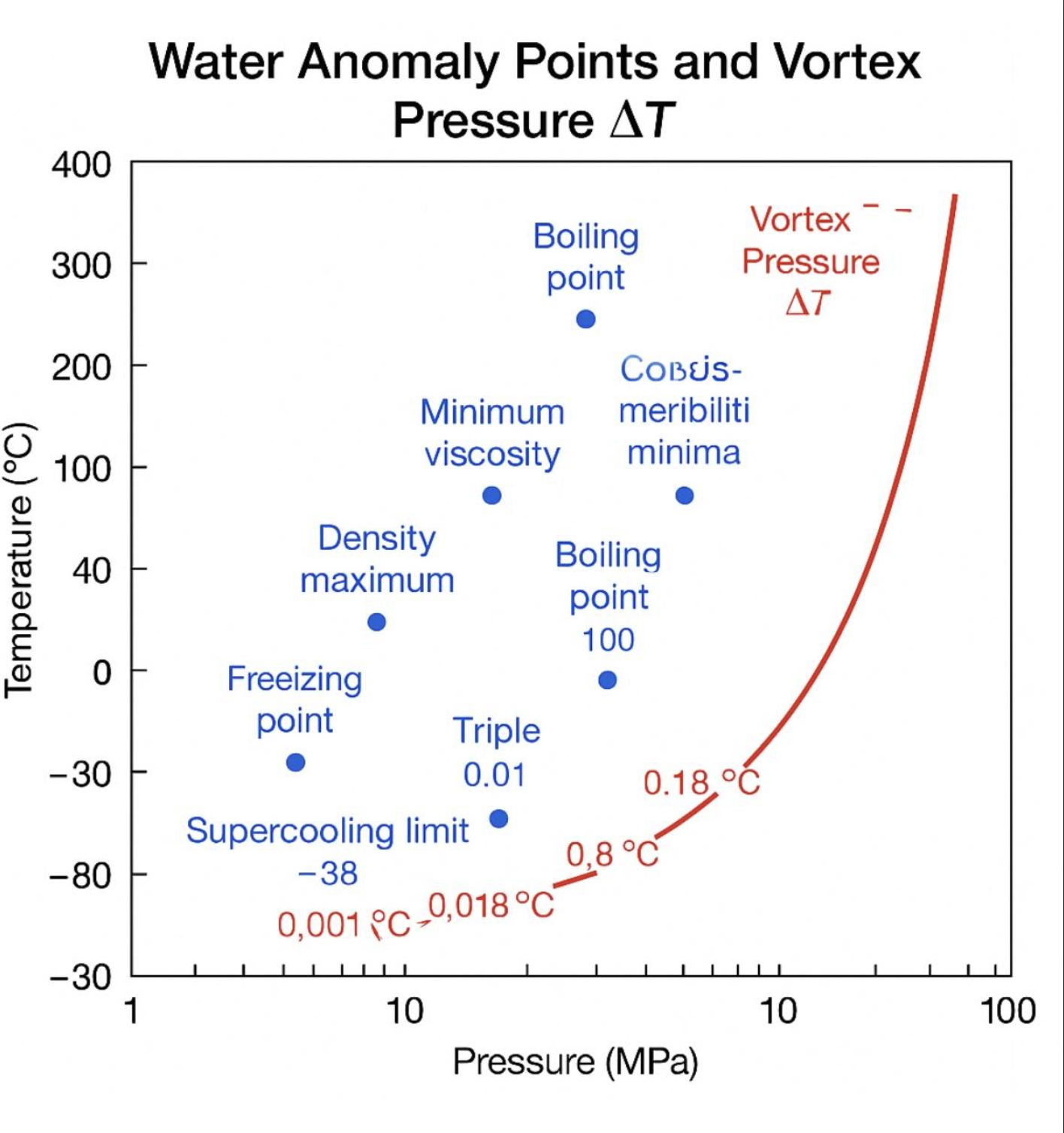
The Anomalies of Water: Beyond Freezing and Boiling Water is one of the most studied substances on Earth, yet it continues to surprise scientists with behaviors that defy the patterns of most liquids. While the freezing point at 0°C (32°F) and the boiling point at 100°C (212°F) are well-known, water has a range of lesser-known anomaly points where its physical properties behave in unusual ways.1. Density Maximum at 4°C (39.2°F)Unlike most substances that become denser as they cool, pure water reaches its maximum density at 4°C. Cooling it further causes it to expand, which is why ice floats. This anomaly plays a critical role in aquatic life survival, as lakes and rivers freeze from the top down.2. Minimum Viscosity at \~30°C (86°F)Water’s viscosity decreases with rising temperature until about 30°C, after which it follows a more typical liquid behavior. This property influences biological fluid flow and industrial processes.3. Compressibility Minima Around 46°CIsothermal compressibility, a measure of how much volume changes under pressure, reaches an unusual minimum at about 46°C. Above and below this temperature, water becomes more compressible.4. Speed of Sound Maximum (\~74°C / 165°F)The speed of sound in water is highest at around 74°C at normal atmospheric pressure. This is due to changes in molecular structure and hydrogen bonding as temperature increases.5. Thermal Expansion Coefficient ShiftFrom 0°C to 4°C, water has a negative thermal expansion coefficient, meaning it contracts when heated. Above 4°C, it follows the typical pattern of expansion with heat.6. Supercooling to –38°CIn extremely pure and undisturbed conditions, water can remain liquid well below its normal freezing point, supercooling to about –38°C before spontaneously freezing.7. Critical Point at 374°C and 218 atmAt the critical point, the distinction between liquid and vapor disappears. Above 374°C and 218 atmospheres of pressure, water becomes a supercritical fluid with unique solvating properties.8. Triple Point at 0.01°C and 611.657 PaAt this precise temperature and pressure, water exists simultaneously as a solid, liquid, and gas in equilibrium.9. Boiling Point Shift Under PressureDue to strong hydrogen bonding, water’s boiling point rises rapidly with pressure. For example, at 2 atmospheres it boils at about 121°C, and at the critical pressure it reaches 374°C. |
|
| CONTACT TEL: 1-608-238-6001 Email: greg@infinityturbine.com | AMP | PDF |