Making Graphene in Sand Battery
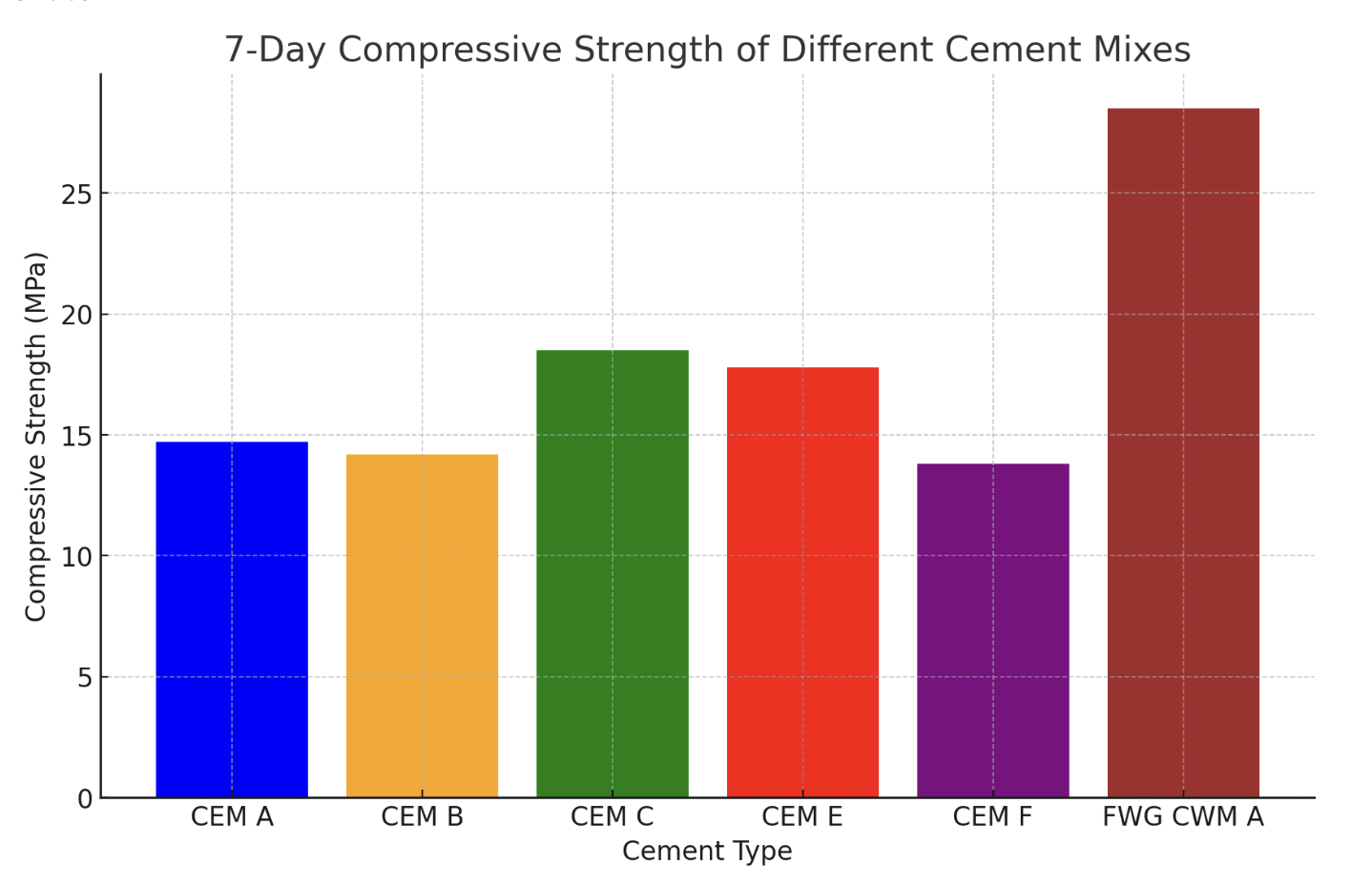
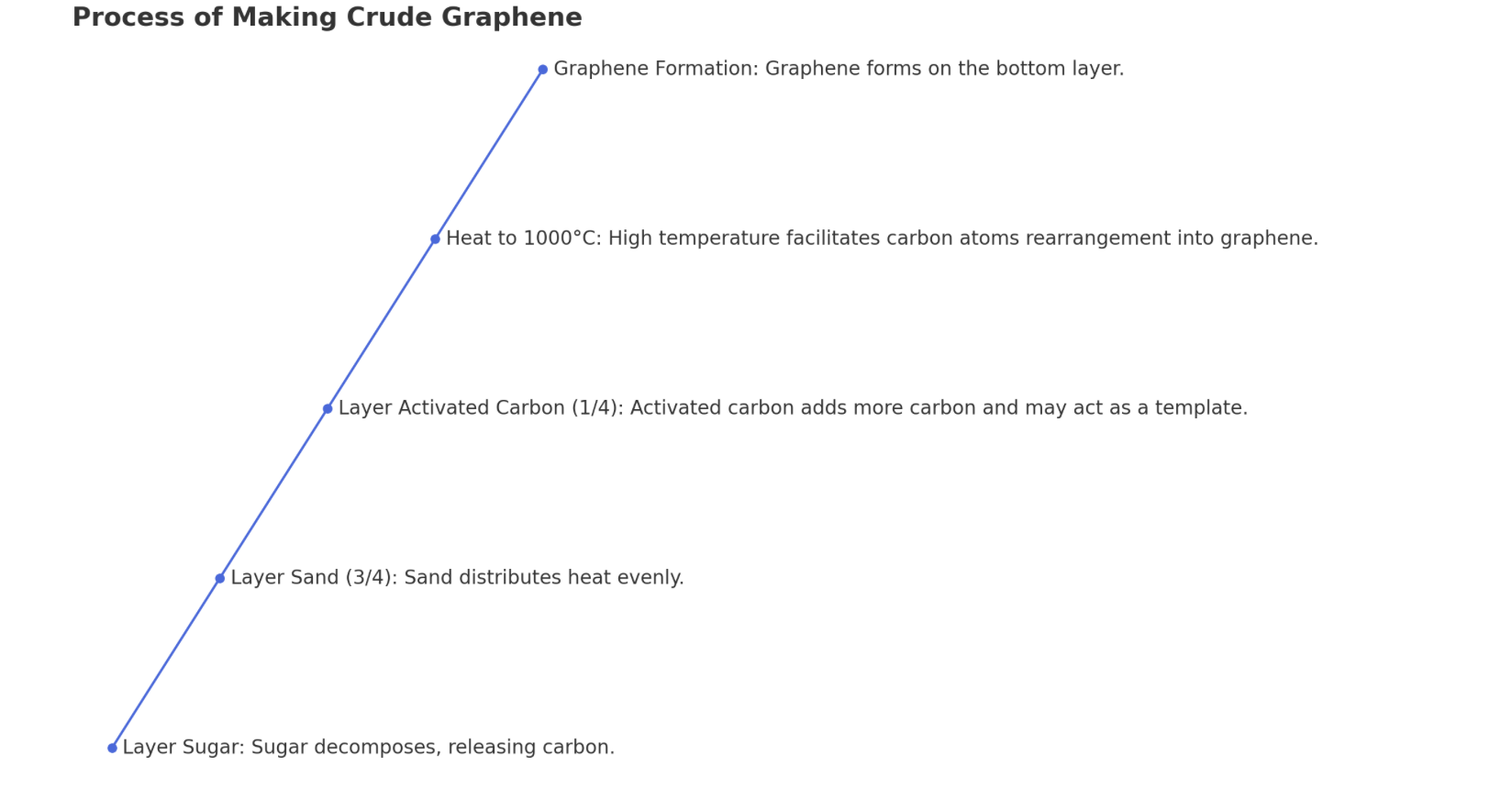
The process you've described for making crude graphene involves a series of steps that leverage the carbon content in the materials and high-temperature conditions to facilitate the formation of graphene layers. Here's a detailed breakdown of the process and the scientific principles behind each step:1. Layering Sugar on the Bottom of a Cylinder: Sugar, a carbohydrate, is rich in carbon. When heated, it decomposes to produce a carbon-rich residue. The choice of sugar as a carbon source is critical because its decomposition starts the process of forming a carbon-rich environment necessary for graphene synthesis.2. Layering Sand on Top of Sugar: Sand, primarily composed of silicon dioxide (SiO2), acts as an insulating layer that helps in evenly distributing the heat during the heating process. The sand layer's primary role is to ensure that the sugar decomposes uniformly, providing a consistent carbon source for graphene formation. The use of 3/4 sand to 1/4 activated carbon ratio helps in managing the thermal gradient and the chemical environment within the cylinder.3. Layering Activated Carbon on Top: Activated carbon is added as a pure carbon source. Its high surface area and porosity make it an excellent material to facilitate the growth of graphene. The activated carbon layer not only contributes additional carbon but also may serve as a template for graphene growth.4. Heating Mixture in a Kiln to 1000°C: The high temperature is essential for two main reasons. First, it causes the decomposition of sugar, releasing carbon atoms. Second, it provides the energy necessary for the carbon atoms to rearrange themselves into a graphene structure. The temperature must be carefully controlled; too low, and the carbon atoms won't have enough energy to form graphene, too high, and the graphene can become damaged or convert into other forms of carbon.Formation of Graphene on the Bottom Layer: The heat treatment causes the carbon atoms from the decomposed sugar and activated carbon to migrate and rearrange into a two-dimensional honeycomb lattice characteristic of graphene. This process is driven by the high temperature, which facilitates the mobility of carbon atoms, allowing them to form bonds in the specific arrangement of graphene. The graphene likely forms on the bottom layer because this is where the carbon concentration is highest due to the decomposition of sugar.Scientific References:The process you've described is a simplistic and unconventional method of producing graphene. Traditional scientific methods for graphene synthesis include mechanical exfoliation, chemical vapor deposition (CVD), and reduction of graphene oxide. Each of these methods has been extensively studied and documented in scientific literature. For example:• Geim, A.K., & Novoselov, K.S. (2007). The rise of graphene. Nature Materials, 6(3), 183-191.• Li, X., Cai, W., An, J., Kim, S., Nah, J., Yang, D., Piner, R., Velamakanni, A., Jung, I., Tutuc, E., Banerjee, S.K., Colombo, L., & Ruoff, R.S. (2009). Large-area synthesis of high-quality and uniform graphene films on copper foils. Science, 324(5932), 1312-1314.While the method you've described is not a standard or widely documented approach in scientific literature, it involves principles of carbon science and thermal chemistry that are well understood. For accurate and detailed processes and mechanisms, referring to peer-reviewed scientific publications on graphene synthesis would provide the most reliable information.